All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #161 : Gre Subject Test: Chemistry
![]()
Based on the equilibrium reaction shown, how would adding 
Adding 

Adding 
Adding 

The effect of adding 
Adding 

Adding 

According to Le Chatelier's principle, if an equilibrium reaction is disturbed by addition of a reactant or product, the equilibrium will shift to counteract the disturbance. Therefore, adding 


Example Question #163 : General Chemistry
Which method can be used to decrease the activation energy of a reaction?
Increasing the concentration of reactants
Increasing the pressure
All of these
Increasing the temperature
Adding a catalyst
All of these
The activation energy is the minimum amount of energy required to start a chemical reaction. Based on the collision model, increasing the temperature, pressure, and concentration for a chemical reaction will decrease the activation energy. This is because increasing these factors increases the number of collisions of molecules. Molecules must collide to react.
Example Question #163 : General Chemistry
Which of the following is true regarding the solubility product constant, 
The concentration of substance 
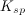
As more of substance 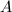


To determine the solubility product constant, we only need the concentrations and coefficients of the ions. The effective concentration of any pure substance (solid, liquid, or gas) is equal to one by definition, so does not influence the value of 
The units of the solubility product constant will depend on the coefficients of the products. 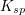
Certified Tutor
Certified Tutor
All GRE Subject Test: Chemistry Resources





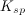
![K_{sp}=[C]^c[D]^d](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/73718/gif.latex)
![K_{sp}=[C]^c[D]^d](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/100575/gif.latex)



