All College Chemistry Resources
Example Questions
Example Question #1 : Titrations
![]()
Determine the volume in mL of 





Determine the moles of 
At the equivalence point of the titration:
Convert the moles of 
Convert the liters to milliliters:
Example Question #1 : Titrations
![]()








Using the concentration of 

At the equivalence point of the titration:
Example Question #2 : Titrations
![]()
Household vinegar contains the organic compound acetic acid with chemical formula, 







At the equivalence point of the titration:
Convert the moles of 
Using the density of 
Determine the percentage of 

Example Question #4 : Titrations
![]()








Using the concentration of 

At the equivalence point of the titration:
Example Question #1 : Titrations
![]()
Household vinegar contains the organic compound acetic acid with chemical formula, 







Convert the liters of 
At the equivalence point of the titration:
Convert the moles of 
Using the density of 
Determine the percentage of 

Example Question #1 : P H
Determine the pH of a solution that is 
Since 

Thus, ![[H_3O^+]=0.40M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668813/gif.latex)
Recall how to find the pH of a solution:
Plug in the given hydronium ion concentration to find the pH of the given solution.
Remember to maintain the correct number of significant figures.
Example Question #2 : P H
Find the pH for a solution that is 


Start by assuming that there is 
Next, find the mass of 
Now, find the number of moles of 
Since we initially assumed that we had 

Since 

Recall how to find the pH of a solution.
Example Question #11 : Acid Base Reactions
Find the pH of a solution that is 

Start by assuming that there is 
Next, find the mass of 
Now, find the number of moles of 
Since we initially assumed that we had 

Since 

Recall how to find the pH of a solution.
Example Question #12 : Acid Base Reactions
Which of the following techniques will decrease the pH of a solution?
Decreasing the concentration of protons
Increasing the amount of Hydroxide molecules
Adding more acid at the same Molarity of the solution
Increasing the amount of solvent
Increasing the concentration of protons
Increasing the concentration of protons
Increasing the concentration of protons of a solution will make the solution more acidic; therefore, it lowers the solution’s pH. Decreasing the concentration of protons will make the solution more basic, raising the pH. Adding more acid of the same molarity of the original solution will not increase the concentration of protons and will not increase acidity or lower the pH. Increasing the amount of hydroxide ions will make the solution more basic and raise the pH. Increasing the amount of solvent will lower the concentration, affecting molarity and not lowering the pH.
Example Question #1 : Henderson Hasselbalch Equation
What is the 


Note: 
In this question, we're given the concentrations of both a weak acid and its conjugate base in solution. We're also provided with the acid-dissociation constant for this acid, and we're asked to find the pH of the solution.
The easiest way to go about solving this problem is to first convert the acid-dissociation constant given into pKa. Then, we can use the Henderson-Hasselbalch equation to solve for the pH of this solution.
Now that we have the pKa, we can use it, along with the concentrations of the weak acid and its conjugate base, in order to solve for the pH of the solution.
Certified Tutor
Certified Tutor
All College Chemistry Resources



















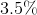





















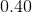



![\text{pH}=-\log([H_3O^+])](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668814/gif.latex)








![[\text{HBr}]=0.06491M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668764/gif.latex)
![[\text{HBr}]=[H_3O^+]=0.06491M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668767/gif.latex)
![\text{pH}=-\log([H_3O^+])](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668768/gif.latex)








![[\text{HI}]=0.3995M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668687/gif.latex)
![[\text{HI}]=[H_3O^+]=0.3995M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668690/gif.latex)
![\text{pH}=-\log([H_3O^+])](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668691/gif.latex)













