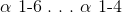All Biochemistry Resources
Example Questions
Example Question #1 : Biochemistry
A solution is prepared from 20mL of 0.25M HCl that is added to 1L of water.
What is the pH of this solution?
HCl is a strong acid so it will completely dissociate into hydrogen ions in an aqueous solution.
Remember that
To find [H+], first find the number of moles of H+ in the solution. Because we are dealing with a monoprotic strong acid, the concentration of hydrogen ions will be equal to the concentration of acid.
Find [H+] by dividing the moles of hydrogen ions by the volume of solution (1L).
Use this value in the original equation for pH.
Example Question #1 : Biochemistry
To overcome an energy barrier between reactants and products, energy must be provided to get the reaction started. This energy, which is recovered as the reaction proceeds, is called:
reaction energy
kinetic energy
initiation energy
activation energy
activation energy
During more reactions, the reactants join to create a transitional form, or transition state, before becoming products. In a spontaneous reaction, the products will always have lower energy than the reactants, but the transition state is almost always higher energy than the reactants. Thus, in order to begin the reaction, energy must be provided to create the transition state from the given reactants. This initial energy is known as the activation energy.
The primary role of enzymes and catalysts in chemical processes is to lower the energy of the transition state, thus lowering the activation energy of the reaction and increasing its rate.
Example Question #2 : Biochemistry
At the end of glycolysis, each molecule of glucose has yielded two molecules of __________, two molecules of __________, and a net of two molecules of __________.
pyruvate . . . NADH . . . ATP
CO2 . . . NAD+ . . . ADP
H2O . . . CO2 . . . ATP
FAD . . . NAD+ . . . ADP
pyruvate . . . NADH . . . ATP
The primary role of glycolysis is to generate pyruvate from glucose. Pyruvate is then converted to acetyl-CoA before entering the Krebs cycle, the next step in cellular metabolism. Two molecules of pyruvate are created from each molecule of glucose.
In order to initiate this process, the reaction consumes two molecules of ATP and converts them to ADP. Later in the process, however, four molecules of ATP are generated. This gives a net yield of two ATP.
Two molecules of NADH are also created during glycolysis. These molecules are the reduced form of NAD+, loosely carrying an additional electron. This electron is donated to the electron transport chain later in cellular metabolism. It will be passed along the proteins of the electron transport chain in order to generate the proton gradient necessary for the function of ATP synthase.
Example Question #1 : Biochemistry
A research scientist is studying a novel enzyme X and wants to characterize this new enzyme. The velocity of the reaction is measured with different substrate concentrations to give the following data:
Determine the approximate 

A rough sketch of the reaction rate against the substrate concentration will show a curve that starts with a large slope, but then levels off to a horizontal asymptote.
Enzyme X follows Michaelis-Menton kinetics. 


Enzyme X is not allosteric because it follows Michaelis-Menton kinetics (curve of that shape). Allosteric enzymes would give a sigmoidal curve.
Example Question #3 : Biochemistry
Glycogen is a polymer of glucose that is held together by __________ bonds. The branch points are held together by __________ bonds.





Example Question #1 : Help With The Electron Transport Chain
What is the purpose of coenzyme Q10 during the electron transport chain?
Regulate the function of ATP synthase
Move electrons from complex I or II to complex III
Carry protons from the mitochondrial matrix into the intermembrane space
Bring oxygen to the end of the electron transport chain to accept electrons
Move electrons from complex I or II to complex III
Coenzyme Q10 is a fat-soluble molecule that facilitates the transfer of electrons from complex I or II to complex III in the electron transport chain. The mobility of coenzyme Q10 in the membrane allows for this unique function. Each complex in the membrane is then able to use the donated electron to push protons into the intermembrane space, generating the gradient that will eventually be used to synthesize ATP.
Coenzyme Q10 does not directly facilitate the movement of protons. Rather, it aids in the transfer of electrons to initiate the process that allows for proton movement. Coenzyme Q10 is also not involved with the regulation of ATP synthase or with bringing oxygen to the electron transport chain.
Example Question #1 : Biochemistry And Metabolism
What type of enzyme is responsible for initiating the process of glycolysis?
Hydrolase
Phosphotase
Kinase
Phosphorylase
Kinase
The initial reactants for glycolysis are glucose, ATP, ADP, and NAD+. The final products are pyruvate, ATP, ADP, and NADH. To get from glucose to pyruvate, a number of enzymes are needed. While knowing the names of each enzyme is not usually necessary, it is important to have a general understanding of the glycolytic process. The first step is phosphorylation of the reactant glucose, which is accomplished by hexokinase in most cells, and by glucokinase in the liver and pancreas specifically. The resultant glucose-6-phosphate then continues through the remaining steps in glycolysis to produce pyruvate.
Example Question #1 : Krebs Cycle
If the Krebs cycle is overstimulated, the body will produce too much of which of the following molecules?
Oxygen
Acetyl CoA
Glucose
Pyruvate
Carbon dioxide
Carbon dioxide
Of the answer choices, only carbon dioxide is a product of the Krebs cycle. If the cycle is overstimulated, too much of the products will be formed and the body will have too much carbon dioxide.
Glucose is the reactant that fuels glycolysis to produce pyruvate, which is then converted to acetyl CoA for the Krebs cycle. As such, each of these would be depleted as reactants fueling an overstimulation of the Krebs cycle.
Example Question #301 : Organic Chemistry, Biochemistry, And Metabolism
Cellular respiration is the set of metabolic reactions that occur in cells to produce energy in the form of ATP. During cellular respiration, high energy intermediates are created that can then be oxidized to make ATP. During what stage are these intermediates produced?
Oxidative phosphorylation
Oxidative phosphorylation and the citric acid cycle
Glycolysis
The citric acid cycle
The citric acid cycle and glycolysis
The citric acid cycle and glycolysis
The citric acid (Krebs) cycle and glycolysis yield high energy intermediates that can then be used to make ATP. Each turn of the citric acid cycle generates NADH and FADH2, and each cycle of glycolysis generates NADH. These intermediates can then donate their electrons and become oxidized in the electron transport chain. Production of these electron donors is essential to the function of the electron transport chain.
Example Question #1 : Biochemistry
Which of the following products cannot be directly formed from pyruvate?
Acetyl-CoA
Acetaldeyde
Lactic acid
None of these can be formed from pyruvate
Ethanol
Ethanol
Pyruvate can be decarboxylated to make acetyl-CoA. This is the process that initiates the citric acid cycle. Pyruvate can also undergo fermentation, and be reduced to either lactic acid or acetaldehyde. Acetaldehyde can then be reduced to ethanol, however, pyruvate cannot directly be converted to ethanol.
Certified Tutor
All Biochemistry Resources





![pH = -log[H^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/181908/gif.latex)


![pH=-log[H^+]=-log(0.005M)](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/181911/gif.latex)