All AP Chemistry Resources
Example Questions
Example Question #81 : Acid Base Reactions
In the following reaction, which is the conjugate acid?
HCO3– + HCl → H2CO3 + Cl–
HCl
H2CO3
HCO3–
Cl–
H2CO3
Conjugate acid has one more H+ than the compound with which it is being compared. Thus, H2CO3 is the conjugate acid of HCO3–.
Example Question #81 : Acid Base Reactions
Which of the following is not true of a neutralization reaction?
It is a specific double-displacement reaction.
There is a reaction between salt and water.
There is a reaction between acid and base.
It has 2 reactants and 2 products.
There is a reaction between salt and water.
The PRODUCTS of a neutralization reactions are salt and water, not the reactants. The rest of the options all correctly pertain to neutralization reactions.
Example Question #82 : Acid Base Reactions
Which of the following chemical groups is expected to be found in a base?
Carboxyl
Bicarbonate
Chloride
Hydroxide
Hydrogen
Hydroxide
Bases can be defined as species that quench hydrogen ions from a solution. A hydroxide ion and a hydrogen ion combine to form water in solution. Recall that basis solutions range in pH from about 7 to 14.
Example Question #83 : Acid Base Reactions
Which of the following groups is expected to be present in an acid?
Carboxyl
Hydroxide
Chloride
Bicarbonate
Hydrogen
Hydrogen
Acids can be defined as species that donate hydrogen ions to solutions. If there is a hydrogen group on a molecule, it is possible that it may be donated to the solution, which will result in a decrease in pH.
Example Question #112 : Reaction Types
Which compound can be both a Bronsted-Lowry acid and Bronsted-Lowry base?
The Bronsted-Lowry definition of an acid is a substance that can donate a hydrogen ion and forms its conjugate base; a Bronsted-Lowry base accepts a hydrogen ion and forms its conjugate acid. Thus we are looking for a substance that can either donate or accept a hydrogen ion (amphoteric). Bisulfite may give up a proton to become 

Example Question #972 : Ap Chemistry
Which of the following is a Lewis acid, but not a Bronsted-Lowry acid?
For this question, we'll need to understand the different definitions of an acid in order to answer it. There are three definitions of acids that are important to know.
1. Arrhenius acids
These are compounds that, when added to water, increase the concentration of 
2. Bronsted-Lowry acids
These are any acid that can release 
3. Lewis acids
This is the most general definition of acids. It is any compound that can accept a lone electron pair.
Lewis acids are the most general kind of acids, meaning that any acid that is Bronsted-Lowry or Arrhenius will also be a Lewis acid. However, the reverse is not true. Not all Lewis acids will fall under the category of Bronsted-Lowry or Arrhenius.
The correct answer in this question is aluminum chloride. We can see that, based on aluminum's position in the periodic table, it has three valence electrons in its outer shell. Each of these electrons is tied up in a shared bond with a chloride. This means that the aluminum in aluminum chloride has six valence electrons. However, since aluminum has a maximum capacity of eight valence electrons, it has room for two more. This vacancy allows the aluminum component of aluminum chloride to accept an electron pair from any sort of electron donor. Thus, aluminum chloride qualifies as a Lewis acid. However, aluminum chloride has no way of producing 
Example Question #81 : Acid Base Reactions
Which of the following is a strong acid?
Formic acid
Lactic acid
Nitric acid
Acetic acid
Hydroflouric acid
Nitric acid
This question is simply testing your memorization of strong and weak acids. Of the list, you should recognize that nitric acid is the only strong acid, and the rest of the choices are weak.
Example Question #81 : Acid Base Reactions
Which of the following is what determines the strength of an acid?
Electronegativity values
The Kb
The Ka
Its physical state
How many bonds the central atom makes
The Ka
The Ka is the acid dissociation constant, and thus it is what determines how strong the acid is. Stronger acids dissociate to a greater extent and produce lower pH values.
Example Question #181 : Reactions And Equilibrium
A 1M solution of a monoprotic acid has a pH of 4.6. What is the 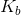
In order to find the base dissociation constant for the conjugate base, we can start by finding the acid dissociation constant for the acid. Since a 1M solution of the acid has a pH of 4.6, we can find the proton concentration of the solution.
Since the acid is monoprotic, we can set the following equilibrium expression equal to its acid dissociation constant.
We can see that, since the acid is monoprotic, the concntration of protons will be equal to the concentration of the acid anion. The final concentration of the acid molecule will be equal to the initial concentration, minus the amount of protons formed. Using these values, we can solve for the equilibrium constant for the acid.
Now that we have the acid dissociation constant, we can find the conjugate base's dissociation constant by setting the product of the two values equal to the autoionization of water.
Example Question #121 : Reaction Types
Would H2SO4 or HNO3 produce a more acidic solution?
H2SO4 since it has a lower pKa
HNO3 since it has a higher pKa
H2SO4 since it has a higher pKa
HNO3 since it has a lower pKa
H2SO4 since it has a lower pKa
Both are strong acids, but H2SO4 is bivalent, realeasing 2 protons for each molecule dissolved in solution. Further, a more acidic solution would have a lower pKa.
Certified Tutor
Certified Tutor
All AP Chemistry Resources










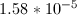



![pH=-\log[H^+]\rightarrow [H^+]=10^{-pH}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/121717/gif.latex)
![\small [H^{+}] = 10^{-4.6} = 2.51*10^{-5}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/94835/gif.latex)
![\small K_{a} = \frac{[H^{+}][A^{-}]}{[HA]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/121718/gif.latex)
![K_a= \frac{[2.51*10^{-5}]^{2}}{[1-2.51*10^{-5}]} = 6.31*10^{-10}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/94836/gif.latex)