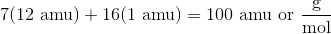All SAT II Chemistry Resources
Example Questions
Example Question #2 : Stoichiometry
What is the approximate percentage of carbon by mass in heptane, which has the molecular formula 
Possible Answers:
Correct answer:
Explanation:
First, calculate the molar mass of heptane, which is
We can calculate the percentage of carbon by mass in heptane by creating a fraction of the mass of carbon over the mass of the entire molecule and converting it into a percentage:
For every 100 g of heptane, 84 g must come from carbon. This means that the percentage of carbon by mass is
Taryn
Certified Tutor
Certified Tutor
University of Florida, Bachelor of Science, Biology, General. University of Florida, Bachelor of Science, Business Administra...
Geo
Certified Tutor
Certified Tutor
Church Teachers College, Bachelor, Science Education. University College of the Carib, Bachelor in Business Administration, B...
All SAT II Chemistry Resources
Popular Subjects
English Tutors in Seattle, MCAT Tutors in Miami, English Tutors in San Francisco-Bay Area, ACT Tutors in Atlanta, Computer Science Tutors in New York City, SSAT Tutors in Chicago, GMAT Tutors in New York City, Biology Tutors in San Diego, ISEE Tutors in Dallas Fort Worth, French Tutors in Los Angeles
Popular Courses & Classes
GMAT Courses & Classes in Boston, Spanish Courses & Classes in San Diego, LSAT Courses & Classes in Seattle, SSAT Courses & Classes in Los Angeles, SAT Courses & Classes in San Francisco-Bay Area, SAT Courses & Classes in Seattle, GMAT Courses & Classes in Denver, GMAT Courses & Classes in Los Angeles, GRE Courses & Classes in San Diego, GRE Courses & Classes in Washington DC
Popular Test Prep
GRE Test Prep in San Francisco-Bay Area, LSAT Test Prep in San Diego, GMAT Test Prep in Houston, GMAT Test Prep in Boston, GMAT Test Prep in Denver, GMAT Test Prep in Dallas Fort Worth, SAT Test Prep in Dallas Fort Worth, GMAT Test Prep in Chicago, GMAT Test Prep in Miami, GMAT Test Prep in Los Angeles