All SAT II Chemistry Resources
Example Questions
Example Question #1 : Molecules
Based on VSEPR theory and electron configurations, which of the following molecules is not matched with its correct geometry?
Possible Answers:
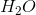




Correct answer:

Explanation:

Example Question #2 : Molecules
What is the molecular geometry of 
Possible Answers:
Trigonal pyramidal
Square pyramidal
Square planar
Tetrahedral
Octahedral
Correct answer:
Square pyramidal
Explanation:
There are six electron domains in 


Taryn
Certified Tutor
Certified Tutor
University of Florida, Bachelor of Science, Biology, General. University of Florida, Bachelor of Science, Business Administra...
Geo
Certified Tutor
Certified Tutor
Church Teachers College, Bachelor, Science Education. University College of the Carib, Bachelor in Business Administration, B...
All SAT II Chemistry Resources
Popular Subjects
SAT Tutors in San Diego, Chemistry Tutors in Houston, Biology Tutors in Chicago, Math Tutors in Atlanta, SSAT Tutors in Washington DC, English Tutors in Dallas Fort Worth, Computer Science Tutors in Atlanta, Statistics Tutors in Chicago, Physics Tutors in New York City, MCAT Tutors in Atlanta
Popular Courses & Classes
SSAT Courses & Classes in Dallas Fort Worth, ACT Courses & Classes in Boston, ACT Courses & Classes in Denver, ACT Courses & Classes in Los Angeles, ISEE Courses & Classes in San Diego, Spanish Courses & Classes in Boston, GMAT Courses & Classes in Phoenix, SAT Courses & Classes in Washington DC, GMAT Courses & Classes in New York City, GRE Courses & Classes in San Francisco-Bay Area
Popular Test Prep
ACT Test Prep in Denver, GMAT Test Prep in Boston, SAT Test Prep in San Francisco-Bay Area, GRE Test Prep in Washington DC, MCAT Test Prep in Philadelphia, GMAT Test Prep in New York City, GMAT Test Prep in Houston, GMAT Test Prep in Phoenix, ACT Test Prep in Atlanta, MCAT Test Prep in New York City




