All Physical Chemistry Resources
Example Questions
Example Question #1 : Solids And Liquids
An unknown molecule (molecule A), in its solid phase, is found to have a density of 

The container will be about halfway full with liquid
No liquid will overflow
Solid will not fit in the container
Liquid will overflow
Liquid will overflow
The dimensions of the cubic container are 
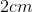

Recall that 


Example Question #2 : Solids And Liquids
Which of the following is true regarding the liquid phase?
I. Liquids have lower entropy than gases
II. Compared to solids, more energy is required to separate liquid molecules
III. Liquids have higher intermolecular forces than gases
I, II and III
III only
I only
I and II
I, II and III
Liquid is an intermediate phase, between solid and gas. Solids are characterized by tightly packed molecules in an organized manner whereas gases are characterized by greatly spread out molecules that are highly disordered; liquids lie somewhere in the middle. This means that they are more disordered than solids (more entropy) and less disordered than gases (less entropy).
The boiling point of a substance is always higher than the melting point; therefore, you always need higher energy to break the interactions between liquid molecules.
The key difference between a liquid and a gas is the distance between the molecules. Liquid molecules are closer together whereas gas molecules are spread apart. Intermolecular forces are the forces between adjacent molecules. Since they are highly spread apart, the gas molecules have lower intermolecular forces.
Example Question #1 : Properties Of Liquids
Which of the following are responsible for the high boiling point of water, compared to molecules of similar size?
Positive charge
Two lone pairs of electrons
Dipole-dipole interactions
Molecular size
Hydrogen bonding
Hydrogen bonding
Hydrogen bonding occurs between water molecules. While intermolecular forces between molecules exist for most molecules, these forces are much weaker than the bond formed between a hydrogen from one molecule and an oxygen, fluorine, and/or nitrogen atom of another molecule. Since the molecules are held more tightly together, more energy is required to break those bonds. This results in a higher temperature required to boil water (boiling breaks bonds between molecules, and this causes the molecules to escape into the gas phase).
Certified Tutor
All Physical Chemistry Resources






