All Organic Chemistry Resources
Example Questions
Example Question #21 : Specific Reactions And Named Reactions
Which of the following best summarizes the haloform reaction?
A methyl ketone is treated with iodine and
The enolate of an ester attacks another ester
The enolate of a dicarbonyl compound attacks a beta carbon of an alkene
An alkyl halide reacts first with the phthalimide ion, then with
A carboxylic acid reacts first with 
A methyl ketone is treated with iodine and
The haloform reaction requires a carbonyl with a terminal alpha carbon. A hydrogen gets abstracted, and the enolate is formed. A halogen attacks the alpha carbon, and the ketone is reformed. This occurs three more times until the carbon has bonds to three halogens. Then the carbon leaves, forming a carbanion, and the base attacks the carbonyl carbon. An ester is formed.
Example Question #653 : Organic Chemistry
Which of the following results in a single ketone product following acid catalyzed hydration?
None of these answers
5-decyne
4-decyne
2-decyne
3-decyne
5-decyne
During acid catalyzed hydration, a hydroxy group replaces one of the bonds in the triple bond and a double bond is formed. This is called an enol. The enol naturally turns into a ketone in a process called tautomerization. The hydroxy group can attach to either carbon across the double bond, and naming is done so that substituents have the lowest numbers. Only on 5-decyne will result in a single product, as no matter which carbon the hydroxy group bonds to, it is still on carbon 5. Thus, the only final product is 5-decone.
The other answer options will still react, but will form multiple products due to lack of symmetry.
Example Question #654 : Organic Chemistry
What is the product of the reaction shown?

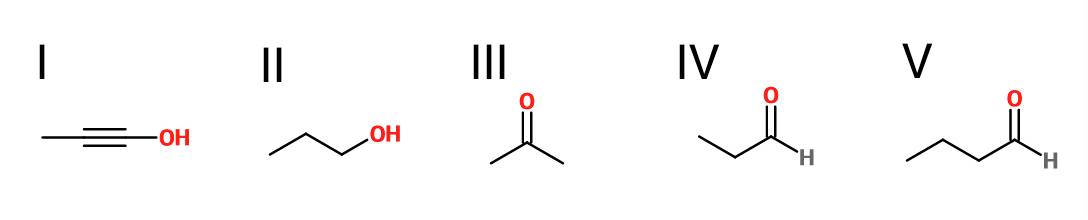
V
III
IV
II
I
IV
First step: bromination across the double bond
Second step: double dehydrohalogenation and removal of terminal alkyne hydrogen
Third step: neutralization of the molecule
Fourth/fifth step: hydroboration/oxidation, followed by keto/enol tautomerization
Example Question #652 : Organic Chemistry
What is a product when propyl-butanoate undergoes saponification?
Propanol
Butyl propanoate
Propanoic acid
None of these
Butanol
Propanol
When naming esters, the root word in the name describes the carbon chain that carries the carbonyl. Saponification describes the breaking up of an ester. This yields an alcohol and a carboxylic acid. The chain with the carbonyl is butyl, so the carboxylic acid will be butanoic acid. That means the side with the alcohol will be propanol.
Example Question #1 : Help With Hydrolysis And Condensation Reactions
Which of the following best summarizes a Claisan condensation?
A carboxylic acid reacts first with 
The enolate of an ester attacks another ester
An alkyl halide reacts first with the phthalimide ion, then with
The enolate of a dicarbonyl compound attacks a beta carbon of an alkene
A methyl ketone is treated with iodine and
The enolate of an ester attacks another ester
The most acidic hydrogen of the ester gets abstracted and the enolate form of the compound is attained. The electrons from the carbon-carbon double bond attack the carbonyl carbon of the other ester. Deesterfication occurs in this same step. The final product has one ketone and one ester.
Example Question #22 : Specific Reactions And Named Reactions
What is the product of the following reaction?

The reaction uses a Grignard reagent's nucleophilic carbon to attack the carbon in carbon dioxide. Following treatment with water the resulting molecule (a carboxylate anion, of the form 
Example Question #1 : Other Reactions

Why does the reaction above require the use of Ag2O?
The Ag2O oxidizes the alcohol to form the alkoxide ion
The Ag2O attacks the alcohol to form the ether
The Ag2O oxidizes the alkyl halide to form the ether
The Ag2O reduces the alcohol to form the alkoxide ion
The Ag2O is an inert solvent and allows the alcohol to attack and from the product without interference
The Ag2O oxidizes the alcohol to form the alkoxide ion
The Ag2O oxidizes the alcohol to form the alkoxide ion. The Ag2O is a reducing agent, and it oxidizes the alcohol because the alcohol loses a proton. Ag2O is a strong base, meaning it will accept protons readily. The given reaction is an example of Williamson ether synthesis. This reaction occurs via an SN2 pathway.
Example Question #2 : Other Reactions
What is the IUPAC name of the following compound?

6-bromo-1-methylcyclohexene
None of the other answers
1-bromo-2-methyl-2-cycloxene
2-bromo-1-methylcyclohexene
3-bromo-2-methylcyclohexene
3-bromo-2-methylcyclohexene
The numbering of the molecule begins across the double bond and goes in the direction where the lowest numbers for substituents can be achieved. Thus, the methyl group will be on carbon two and the bromine on carbon 3. Substituents are ordered alphabetically. The base molecule is a six-membered ring with one double bond. This is called a cyclohexene.
Example Question #2 : Other Reactions
What is the proper name of this molecule?

E-3-methyl-2-pentene
Z-2-isohexene
None of the other answers
E-2-ethyl-2-butene
E-3-methyl-3-pentene
E-3-methyl-2-pentene
The longest chain has five carbons and a double bond, so the base molecule is pentene. There is a methyl group on carbon 3. The double bond is between carbons two and three. The highest priority substituents are the ethyl group on one side and the methyl group on the other. One is up and one is down, so the molecule is E as opposed to Z.
Example Question #3 : Other Reactions
Which of the following best summarizes the Michael reaction?
A methyl ketone is treated with iodine and
A carboxylic acid reacts first with 
The enolate of an ester attacks another ester
An alkyl halide reacts first with the phthalimide ion, then with
The enolate of a dicarbonyl compound attacks a beta carbon of an alkene
The enolate of a dicarbonyl compound attacks a beta carbon of an alkene
The most acidic carbon in a dicarbonyl gets abstracted to form an enolate. The reaction involves a ketone with a double bond between the alpha and beta carbons. The carbon-carbon souble bond electrons attack the beta carbon of the alkene. At this point, the electrons from the alkene move to form a double bond between the carbonyl and alpha carbon and an enolate is fromed. The negative oxygen bonds to a hydrogen, forming the enol. Through enol-ketone tautamerization, a ketone is formed.
All Organic Chemistry Resources















