All Organic Chemistry Resources
Example Questions
Example Question #3 : Help With Alkene Synthesis

What is the major product for the reaction given?





The reason this is the major product is because on tertiary alcohols are best dehydrated based on the E1 mechanism below:

Example Question #601 : Organic Chemistry

What is the major product for the reaction given?





The reason this is the major product is because tertiary alcohols are best dehydrated based on the E1 mechanism below:

Example Question #5 : Help With Alkene Synthesis

What is the major product for the reaction given?





Below is the mechanism for the reaction given to form the alkene:

Example Question #1 : Organic Chemistry
What is the best reagent for abstracting a hydrogen from ethyne?
None of these
The triple bond in ethyne makes the hydrogens slightly more acidic than those found in ethane. A very strong base, such as the conjugate base of ammonia, would be able to abstract that hydrogen. The abstraction turns the base into ammonia. It also creates a carbanion that can be used for chain extension and alkyne synthesis.
Example Question #1 : Alkyne Chemistry

What is the product of the compound when it reacts with two equivalents of base?
None of these
2-pentyne
1-pentyne
1-pentene
2-pentene
2-pentyne
For each equivalent of base, a pi bond is formed between the carbons initially bound to the bromine atoms. For each bond formed, a bromine leaving group leaves the hydrocarbon. One equivalent of base abstracts a hydrogen. The electrons from the bond to the hydrogen create a pi bond. This occurs twice, and a triple bond is formed. The result is a 5-carbon chain with a triple bond between the second and third carbons. This molecule is 2-pentyne.
Example Question #1 : Help With Aldehyde And Ketone Synthesis
What reagents are required to undergo Swern oxidation?
Dichloromethane, trimethylamine, and DMS
Diethyl ether, organometallic compound, and H3O+
COCl2, triethylamine, and DMSO
SOCl2, pyridine, and triethylamine
Pyridine, DMSO, and HCOOH
COCl2, triethylamine, and DMSO
Swern oxidation requires COCl2, triethylamine, and DMSO. The same reaction can be accomplished using PCC and dichloromethane. This reaction oxidizes an alcohol to a carbonyl, but only works with primary and secondary alcohols.
Example Question #1 : Help With Aldehyde And Ketone Synthesis

Which of the following reactions will result in the given product?
D
A
E
B
C
C
The starting material first needs to be brominated, which occur at the tertiary carbon. Then, an elimination reaction needs to take place to form an alkene intermediate, which will transpire through the E2 pathway with the tertbutoxide ion in tertbutanol. After this, an alcohol needs to be formed through hydroboration because we need the carbonyl group on the less subsituted carbon. After an alcohol is formed, the PCC reaction can oxidize the alcohol to make it a carbonyl. All these reactions are shown in choice C.
Example Question #12 : Reactions By Product
A compound, 


What is the original compound?
4-chloro-1,2-dimethylcyclopentane
None of the other answers
2-chloro-1,3-dimethylcylopentane
1-chloro-1,2-dimethylcyclopentane
2-chloro-1,1-dimethylcyclopentane
2-chloro-1,3-dimethylcylopentane
In the first step of the reaction, a chlorine is abstracted and a double bond is formed. In the ozonolysis step, the molecule is broken at the double bond and each carbon at that bond gets double bonded to an oxygen. The presence of zinc keeps it from becoming a carboxylic acid.
Working backwards, you must remove the oxygens from the final product and redraw the molecule at the double bond. This intermediate is 1,3-dimethylcyclopentene. Next, the chlorine must be added to one of the carbons on the double bond. The only possible choice is 2-chloro-1,3-dimethylcyclopentane.
Example Question #2 : Help With Aldehyde And Ketone Synthesis

Which of the following reagents is required to produce the ketone product?
In order to convert a secondary alcohol into a ketone, we must employ 
Example Question #1 : Help With Ether And Ester Synthesis
Which of the following reactions is NOT a valid synthesis of methyl benzoate?
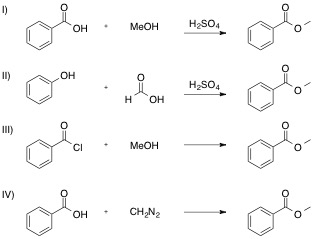
I
All are valid syntheses of methyl benzoate
II
III
IV
II
Each reaction is shown, with its name and correct product below.
If you are unsure how the product is obtained through each reaction, use your textbook or internet sources to find a mechanism for each. Reactions I, II, and III are specific examples of substitution reactions, and the last, reaction IV, goes through a more complex addition-elimination reaction to release nitrogen gas.
Working through the mechanism for a reaction is always a certain way to find the product, even if you don't know from the outset what it will be. Just look for the electrophile and nucleophile in each step of the mechanism, and push those arrows!

All Organic Chemistry Resources