All Organic Chemistry Resources
Example Questions
Example Question #1 : Carboxylic Acid Chemistry
Which one of the following compounds can produce a carboxylic acid only when reacted with sodium dichromate and sulfuric acid?
2-pentanone
1-pentanol
2-methylpentan-2-ol
Pentanal
2-pentanol
1-pentanol
After recognizing the reagents given, we know we need to begin with an alcohol. The alcohol choices differ only by how substituted they are.
For a carboxylic acid to form from a reaction with sodium dichromate and sulfuric acid, a primary alcohol needs to be available. Therefore, 1-pentanol is the correct answer.
Example Question #2 : Help With Carboxylic Acid Synthesis
When 3-chloroheptane undergoes malonic ester synthesis, the final product is __________.
None of the other answers
a ten-carbon ketone
malonic ester
3-ethylheptanoic acid
nonanoic acid
3-ethylheptanoic acid
The malonic ester is deprotonated at the most acidic hydrogen, the one on the carbon between the two oxygens. The electrons from that bond to the hydrogen form a carbon-carbon double bond while electrons from the oxygen-carbon double bond go to the oxygen atom. This is the enolate form. The chlorine leaves the 3-chloroheptane and the electrons from the carbon-carbon double bond bond to the carbon that the chlorine left. At the same time, the carbonyl is reformed. After deesterfication, 3-ethylheptanoic acid is formed.
Example Question #11 : Carbonyl Products
Which of the following is not a derivative of a carboxylic acid?
All of these are carboxylic acid derivatives
Aldehyde
Ester
Amide
Aldehyde
Aldehyde is not a derivative of carboxylic acid. Esters can be derived from carboxylic acids by reacting them with 



Example Question #621 : Organic Chemistry
Which of the following reagents is needed to convert an amide into a carboxylic acid?
All of these
As a general rule, any carboxylic acid derivative can be converted into al carboxylic acid when reacting with
Example Question #13 : Carbonyl Products

What is the product of the given reaction?


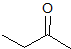



The hydrolyzation of a nitrile with hydronium leads to a carboxylic acid. Only one choice is a carboxylic acid.
Example Question #622 : Organic Chemistry
Please choose the best answer for the following question.
Which of the following reagents is best for converting a primary alcohol to a carboxylic acid?

PCC


Example Question #202 : Gre Subject Test: Chemistry
What is the product of a hydroboration–oxidation reaction with 1-hexylcyclohexene?
Cyclohexane
2-hexylcyclohexanol
Hexylcyclohexane
1-hexylcyclohexanol
3-hexylcyclohexanol
2-hexylcyclohexanol
This reaction is an electrophilic addition reaction with an alkene. This is one of many alkene addition reactions that can add an -OH group onto your starting material. The key aspect of an hydroboration-oxidation reaction is the anti-Markovinikov addition to the double bond. The -OH group should be on the least substituted of the two carbons that originate from the double bond. In light of this information, the answer is 2-cyclohexanol.
Example Question #622 : Organic Chemistry

What is the product when the given starting compound is reacted with lithium aluminum hydride and acid?
1-butanol
1-butanone
2-butanone
No reaction will occur
2-butanol
2-butanol
This reaction involves a very strong reducing agent in lithium aluminum hydride, 
Example Question #1 : Help With Alcohol Synthesis
What reagents are required to efficiently form a tertiary alcohol with two of the same substituents in only two steps?
An ester reacted with: 1) excess ethanol and 2) H3O+
An acid chloride reacted with 1) 2RMgBr and 2) hexane
An ester reacted with 1) 2RMgBr and 2) H3O+
An ester reacted with 1) RMgBr and 2) H3O+
An ester reacted with 1) 2RLi and 2) NH3
An ester reacted with 1) 2RMgBr and 2) H3O+
This is an example of a Grignard reaction that requires the use of an ester or acid chloride, and more than one equivalent of an organometallic. The second step would be to react the product of the first step in an acidic source.
Example Question #12 : Carbonyl Products
When butanoic acid undergoes the Hell-Volhard-Zelinsky (HVZ) reaction, the final product is __________.
3-bromobutanoic acid
2-bromobutanoic acid
None of the other answers
butanoyl bromide
heptanoic acid
2-bromobutanoic acid
In the HVZ reaction of a carboxylic acid, a bromine is added to the alpha carbon. Phosphorus catalyzes the reaction and allows for the formation of an acyl halide. Acyl halides readily undergo enol-ketone tautomerization. The enol form uses electrons from the carbon-carbon double bond to bond to the bromine. In water, the two bromine molecules convert back to a carboxylic acid with one bromine on the alpha carbon.
All Organic Chemistry Resources











