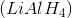All Organic Chemistry Resources
Example Questions
Example Question #7 : Help With Acid Base Reactions
Which of the following sets of bases are listed from most basic to least basic?
The correct ranking from most basic to least basic is:
The best bases are negatively charged, and the worst bases are positively charged (acidic). The stronger the base, the weaker (more stable) it's conjugate acid. An alkane is a very stable conjugate acid, which tells us that 
We know based on charge alone, that 



We know that 




Example Question #53 : Reactions Types
Which of the following sets of acids are correctly listed from most to least acidic?
Remember that the strongest acids have the weakest conjugate bases. 




Example Question #1 : Help With Acid Base Reactions

Rank the given molecules in order of increasing pKa.
IV, II, V, I, III
III, II, V, I, IV
II, V, IV, I, III
III, I, IV, V, II
I, II, V, IV, III
II, V, IV, I, III
Recall that the stronger an acid, the lower the pKa.
II (two fluorine atoms really close to 
V (one fluorine really close to 
I (one fluorine a little further away from 
IV (no inductive effect)
III (alcohols are much less acidic than carboxylic acids, and it has the highest pKa of all)
Example Question #2 : Help With Acid Base Reactions
Which side (left or right) of the following reaction is favored and why?

Right side, because the 


Left side, because the 


Left side, because the 


Right side, because the 


Right side, because the 


The side of the reaction that is favored will have the acid with the higher 

Example Question #151 : Organic Chemistry
Which reagent must be used with hydrobromic acid to convert 1-hexene to 1-bromohexane?
A peroxide
None of these
A peroxide
The double bond in 1-hexene is across carbons one and two. Just adding HBr would put the bromine on carbon 2 because the bromine is more stable on the more highly subtituted carbon. If you want to put the bromine on the less substituted carbon (generating 1-bromohexane), you must carry out the reaction in the presence of peroxides. The mechanism involves a free radical on the secondary carbon and a bromine addition on the primary carbon.
Example Question #152 : Organic Chemistry
Which site would the reagent N-Bromosuccinimide (NBS) be most likely to attack?
The most substituted atom is most likely to be attacked, as a radical intermediate would be created. The radical intermediate would be most stable on the most substituted carbon.
Example Question #82 : Organic Concepts
Which of the following reagents would create the product for the reaction below?










The radical reaction would create the anti-Markovnikov product shown and both 


Example Question #84 : Organic Concepts
Which of these steps in a free-radical halogenation of an alkene will not generate a radical molecule?
None of these steps
Initiation
Termination
All of these steps
Propagation
Termination
Termination is the only step that does not generate a radical product. The termination step ends the reaction by bringing together two radical molecules to produce a stable non-radical product.
Example Question #161 : Organic Chemistry

Classify the type of reaction given.
Substitution reaction
Homolytic bond breaking
Rearrangement reaction
Heterolytic bond breaking
Homolytic bond breaking
Homolytic bond breaking occurs when a molecule breaks up to form two or more new products. In the reaction given, molecular chlorine forms two radicals in which one electron stays with each fragment formed.
Example Question #2 : Other Reactions
Which of the following is not capable of oxidizing a secondary alcohol to a ketone?


Lithium aluminum hydride
Pyridinium chlorochromate (PCC)
All of these answers can oxidize secondary alcohols to ketones
Lithium aluminum hydride
Lithium aluminum hydride is correct because it is a reducing agent, and is therefore not capable of oxidizing secondary alcohols. Instead, LAH could be used to perform the reverse reaction, reducing a ketone to an alcohol. The other answer choices are oxidizing agents.
Certified Tutor
All Organic Chemistry Resources