All Organic Chemistry Resources
Example Questions
Example Question #2 : Identifying Electron Donating Groups
Which of the given compounds is not a Lewis acid?
All of these are Lewis acids
A Lewis acid is an electron pair acceptor. A Lewis base is an electron pair donor.
The carbocation is clearly trying to accept electrons due to the positive charge. 

The correct answer has a lone pair on the nitrogen, and thus has electrons to donate and as a Lewis base. 
Example Question #1 : Identifying Electron Withdrawing Groups
Which of the following R groups would increase the rate of the following substitution reaction?
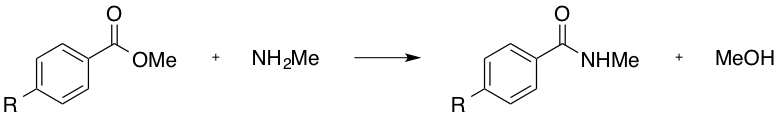
The above reaction would more readily proceed if the electrophilicity of the carbonyl carbon were enhanced. This may be achieved through electron withdrawal via the R group.
The ether (-OMe), the methyl (-Me), and the hydroxyl (-OH), would all produce a electron-donating effect, and are thus incorrect answers.
The nitro group (-NO2), and the positively charged, tetra-substituted amino group (consider the structure once this trimethyl amino group is connected to the aryl ring) are both electron-withdrawing. As the trimethyl amino group will have an overall positive charge (and the nitro group is neutral overall), the trimethyl amino group is the stronger electron-withdrawing moiety, and is thus the correct answer.
Example Question #1 : Help With Addition Reactions
In an addition reaction
one pi bond is broken, and two sigma bonds are formed
none of these
one sigma bond is broken, and one sigma bond is formed
one pi bond is broken, and one pi bond is formed
two sigma bonds are broken, and one pi bond is formed
one pi bond is broken, and two sigma bonds are formed
Addition reactions involve breaking one pi bond (double bond) and forming two sigma bonds in the product.
Example Question #1 : Reactions Types
A compound 

The original compound has no triple bonds.
4 double bonds and 1 ring
3 double bonds and 2 rings
4 double bonds and 2 rings
3 double bonds and 1 ring
None of the other answers
3 double bonds and 2 rings
Hydrogenation of a double bond involves the bond breaking and a hydrogen being added to each carbon of that double bond. You can tell the number of double bonds by taking the number of hydrogens added and dividing it by 2.
6 added hydrogen divided by 2 is 3 double bonds.
A hydrocarbon with zero degrees of unsaturation and 


Example Question #1 : Reactions Types
Which of the following reagents are required to convert 1-pentene to pentane?
Methanol
In order to convert an alkene into an alkane, we need a 
Example Question #91 : Organic Chemistry
Ph = phenyl = benzene
Which reagent would work best to convert 

We can reduce the alkene here by simply adding two hydrogens with 
Example Question #93 : Organic Chemistry

Suppose that the given reactant, 1-hexene, is reacted with 





In this question, we're told that our starting material, 1-hexene, is being reacted with hydrobromic acid in the presence of ultraviolet (UV) light. To solve this, we need to consider how halogens add to alkenes, specifically in the presence of ultraviolet light.
First, it's important to note that UV light will cause the hydrogen and bromine atoms in 
The bromine radical adds to the alkene first at the 1-carbon in an anti-Markovnikov fashion. The reason it adds to this carbon (and not the 2-carbon) is because a secondary free radical is more stable than a primary free radical. Upon formation of this secondary radical, it will react with the hydrogen free radical in solution to generate the finished product, 1-bromohexane.
Example Question #3 : Reactions Types
Which of the following electrophiles is matched incorrectly with its catalyst needed for electrophilic aromatic substitution on a benzene ring?
All are correctly matched
A catalyst is often required for an electrophilic aromatic substitution reaction. All reagents are paired with their correct catalysts except for 


Example Question #1 : Help With Addition Reactions

Classify the type of reaction given.
Substitution
Rearrangement
Addition
Elimination
Addition
An addition reaction is a reaction in which the reactants react to combine and form one product. It is the opposite of an elimination reaction. In the reaction given, the reactants hydrochloric acid and ethylene combine to form the product 1-chloropropane.
Example Question #1 : Reactions Types

Classify the type of reaction given.
Rearrangement
Elimination
Addition
Substitution
Addition
An addition reaction is a reaction in which the reactants react to combine and form one product. It is the opposite of an elimination reaction. In the reaction given, the reactants 2-butene and molecular hydrogen combine to form the product butane.
Certified Tutor
All Organic Chemistry Resources


























