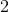All Organic Chemistry Resources
Example Questions
Example Question #131 : Organic Chemistry
The molecule shown reacts with 
These conditions are perfect for an 


Example Question #7 : Help With Elimination Reactions
Chloropropane reacts with 
Through which mechanism did this reaction occur?
The given conditions are perfect for an 


Example Question #42 : Reactions Types
Which of the following hydrogens are removed upon treatment of the pictured molecule with potassium tert-butoxide?

III
I
IV
II
III
Treatment with strong base indicates E2 mechanism. The halide has two beta-carbons (II and III), and potassium tert-butoxide is a bulky base. The favored product is the Hofmann product (less substituted alkene). Therefore III is the hydrogen that's removed.
Example Question #2 : Help With Elimination Reactions
What is the product of the reaction shown?


I, II, and III
II only
I only
III only
I and II
II only
E2 reaction mechanism requires antiperiplanar orientation between the leaving group and the hydrogen. Given the stereochemistry of the methyl group, forming an alkene bond (to give the more substituted product) is impossible.
Example Question #10 : Help With Elimination Reactions
What is the major product in the following reaction?
This is an acid-catalyzed dehydration of a tertiary alcohol. The first step is protonation of the alcohol oxygen which will create a good leaving group (
Example Question #61 : Organic Concepts

Classify the type of reaction given.
Elimination
Rearrangement
Addition
Substitution
Elimination
An elimination reaction occurs when there is a release of atoms in a given compound to produce two or more products. In the reaction given a hydrogen and chloride atom are eliminated from the original compound to form hydrochloric acid and ethylene.
Example Question #41 : Reactions Types

Classify the type of reaction given.
Heterolytic bond breaking
Addition
Substitution
Homolytic bond breaking
Heterolytic bond breaking
Heterolytic bond breaking occurs in polar compounds to form to products of opposite charges. In these types of reaction two electrons from the original bond stays with one fragment upon cleavage. In the reaction given, the bond between the hydrogen and chlorine atom is broken with the two electrons from the original bond staying with the chlorine atom. The resulting products are hydrogen ion and chloride ion.
Example Question #45 : Reactions Types

Classify the type of reaction given.
Elimination
Homolytic
Catalysis
Addition
Elimination
An elimination reaction occurs when there is a release of atoms in a given compound to produce two or more products. In the reaction given a hydrogen and chloride atom are eliminated from the original compound to form one 2-butene, potassium chloride and water molecule.
Example Question #42 : Reactions Types
Which of the following is the correct major product of the above reaction?




Here we see an 
Example Question #1 : Help With Rearrangement Reactions
What is the major product of the reaction shown?


II
IV
V
III
I
IV
This reaction adds 


Certified Tutor
All Organic Chemistry Resources