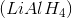All Organic Chemistry Resources
Example Questions
Example Question #161 : Organic Chemistry

Classify the type of reaction given.
Substitution reaction
Homolytic bond breaking
Rearrangement reaction
Heterolytic bond breaking
Homolytic bond breaking
Homolytic bond breaking occurs when a molecule breaks up to form two or more new products. In the reaction given, molecular chlorine forms two radicals in which one electron stays with each fragment formed.
Example Question #161 : Organic Chemistry
Which of the following is not capable of oxidizing a secondary alcohol to a ketone?
Lithium aluminum hydride


Pyridinium chlorochromate (PCC)
All of these answers can oxidize secondary alcohols to ketones
Lithium aluminum hydride
Lithium aluminum hydride is correct because it is a reducing agent, and is therefore not capable of oxidizing secondary alcohols. Instead, LAH could be used to perform the reverse reaction, reducing a ketone to an alcohol. The other answer choices are oxidizing agents.
Example Question #1 : Help With Oxidation Reduction Reactions
In the following equation, which element is reduced?
Manganese, iodine, and oxygen are all oxidized.
In an oxidation-reduction (redox) reaction, reduction and oxidation both occur. Thus, not all of the elements can be oxidized, and not all of them can be reduced. In this equation, the oxidation number of oxygen is 










At the end of the reaction, 



Thus, 




Example Question #1 : Organic Functional Groups And Molecules
Which compound(s) shown above is(are) aromatic?
A and C
D
A, B, and C
A
A
For a compound to be considered aromatic, it must be flat, cyclic, and conjugated and it must obey Huckel's rule. Huckel's rule states that an aromatic compound must have 
Example Question #1 : Organic Functional Groups And Molecules
Which of the compounds below is antiaromatic, assuming they are all planar?
(2) Annulene
(8) Annulene
(6) Annulene
(14) Annulene
(10) Annulene
(8) Annulene
The correct answer is (8) Annulene. This is because all aromatic compounds must follow Huckel's Rule, which is 4n+2. Note that "n" in Huckel's Rule just refers to any whole number, and 4n+2 should result in the number of pi electrons an aromatic compound should have. For example, 4(0)+2 gives a two-pi-electron aromatic compound.
It is also important to note that Huckel's Rule is just one of three main rules in identifying an aromatic compound. An annulene is a system of conjugated monocyclic hydrocarbons. A compound is considered anti-aromatic if it follows the first two rules for aromaticity (1. Pi bonds are in a cyclic structure and 2. The structure must be planar), but does not follow the third rule, which is Huckel's Rule.
(8) Annulene follows the first two rules, but not Huckel's Rule, and is therefore antiaromatic; no value of a whole number for "n" will result in 8 with the formula 4n+2.
Example Question #1 : Organic Functional Groups And Molecules
Which of the following best describes the given molecule?
None of these
Aromatic
Non-aromatic
Anti-aromatic
Anti-aromatic
A molecule is anti-aromatic when it follows all of the criteria for an aromatic compound, except for the fact that it has 

Example Question #1 : Identifying Aromatic Compounds
Which of the following best describes the given molecule?
Aromatic
Anti-aromatic
Non-aromatic
None of these
Non-aromatic
A molecule is aromatic when it adheres to 4 main criteria:
1. The molecule must be planar
2. The molecule must be cyclic
3. Every atom in the aromatic ring must have a p orbital
4. The ring must contain 
The carbon on the left side of this molecule is an sp3 carbon, and therefore lacks an unhybridized p orbital. The molecule is non-aromatic.
Example Question #96 : Organic Concepts

How many pi electrons does the given molecule have?
There are 14 pi electrons because oxygen must contribute 2 pi electrons to avoid antiaromaticity. The other 12 pi electrons come from the 6 double bonds.
Nitrogen does not contribute any pi electrons, as it is 
Example Question #1 : Organic Functional Groups And Molecules

How many pi electrons does the given compound have?
If oxygen contributes any pi electrons, the molecule will have 12 pi electrons, or 4n pi electrons, and become antiarmoatic. As it is now, the compound is antiaromatic.
Nitrogen cannot give any pi electrons because it's lone pair is in an sp2 orbital. Boron has no pi electrons to give, and only has an empty p orbital.
Example Question #1 : Organic Functional Groups
Consider the molecular structure of anthracene, as shown below.

Which of the following is true regarding anthracene?
Anthracene follows Huckel's rule
All of these answer choices are true
There is an even number of pi electrons
Anthracene is planar
All of these answer choices are true
In this question, we're presented with the structure of anthracene, and we're asked to find which answer choices represent a true statement about anthracene. Let's go through each of the choices and analyze them, one by one.
First, let's determine if anthracene is planar, which is essentially asking if the molecule is flat. When looking at anthracene, we see that the molecule is conjugated, meaning there are alternating single and double bonds. If we look at each of the carbons in this molecule, we see that all of them are 

Now let's determine the total number of pi electrons in anthracene. Remember, pi electrons are those that contribute to double and triple bonds. So, we'll need to count the number of double bonds contained in this molecule, which turns out to be 

Lastly, let's see if anthracene satisfies Huckel's rule. This rule is one of the conditions that must be met for a molecule to be aromatic. It states that when the total number of pi electrons is equal to 

Since we arrived at an integer value for 
All of the answer choices are true statements with regards to anthracene.
Certified Tutor
All Organic Chemistry Resources