All Organic Chemistry Resources
Example Questions
Example Question #141 : Organic Chemistry

Classify the type of reaction given.
Heterolytic bond breaking
Homolytic bond breaking
Addition
Substitution
Heterolytic bond breaking
Heterolytic bond breaking occurs in polar compounds to form to products of opposite charges. In these types of reaction two electrons from the original bond stays with one fragment upon cleavage. In the reaction given, the bond between the hydrogen and chlorine atom is broken with the two electrons from the original bond staying with the chlorine atom. The resulting products are hydrogen ion and chloride ion.
Example Question #142 : Organic Chemistry

Classify the type of reaction given.
Elimination
Homolytic
Addition
Catalysis
Elimination
An elimination reaction occurs when there is a release of atoms in a given compound to produce two or more products. In the reaction given a hydrogen and chloride atom are eliminated from the original compound to form one 2-butene, potassium chloride and water molecule.
Example Question #42 : Reactions Types
Which of the following is the correct major product of the above reaction?




Here we see an 
Example Question #141 : Organic Chemistry
What is the major product of the reaction shown?


V
III
IV
II
I
IV
This reaction adds 


Example Question #51 : Reactions Types
What is the major product of the reaction shown?


III
II
I
None of these
IV
II
After carbocation is formed, a rearrangement reaction stabilizes positive charge by putting it on a tertiary carbon. This is done by a methyl shift. Recall that tertiary carbocations are the most stable due to the inductive effect of alkyl groups on the electron-deficient carbocation.
Example Question #52 : Reactions Types
What is the major product of the reaction shown?
The first step of this reaction will be protonation of the hydroxyl oxygen to create a good leaving group. When the leaving group leaves, what's left is a secondary carbocation that is vicinal to (next to) a quaternary carbon. A methyl shift is thermodynamically favored in this case, as the rearrangement will leave a tertiary carbocation. Following the rearrangement the nucleophile (bromide) will attack the tertiary carbocation, forming a sigma bond with the carbon. The answer is thus 
Example Question #1 : Acid Base Reaction
For which of the following acid-base reactions will the equilibrium lie on the left side?

The pKa value indicates how strong an acid is, and acid strength increases as pKa decreases. The side of a reaction with a lower pKa is going to dissociate more, pushing the equilibrium over to the other side. The equilibrium will thus lie on the side with the HIGHER pKa.
Since the pKa of acetic acid (4.76) is higher than the pKa of trifluoroacetic acid (0), the reaction will shift to the left to reach equilibrium.
Example Question #71 : Organic Concepts
A carboxylic acid has a pKa of 5. At a pH of 8, what is the ratio of salt to acid?
Use the Henderson Hasselbalch equation:
Example Question #72 : Organic Concepts

List the given compounds in order of decreasing basicity.
III, II, IV, I
IV, I, III, II
II, I, III, IV
II, III, IV, I
II, III, I, IV
II, I, III, IV
An easy way to consider relative base strengths is to consider the strength of the compounds' conjugate acids.The stronger the conjugate acid, the weaker the base. Water (compound IV) is the least basic of the compounds because its conjugate acid, 






Example Question #51 : Reactions Types

Rank these weak acids by decreasing 
IV, III, I, II
II, III, I, IV
IV, I, II, III
III, IV, I, II
III, IV, II, I
III, IV, II, I
The governing principle regarding the prediction of 




All Organic Chemistry Resources








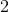










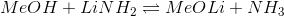




![pH = pKa + log \frac{[salt]}{[acid]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/439185/gif.latex)
![8 = 5 + log \frac{[salt]}{[acid]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/439186/gif.latex)
![10^{3} = \frac{[salt]}{[acid]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/439187/gif.latex)




