All Organic Chemistry Resources
Example Questions
Example Question #231 : Organic Concepts
Rank the given molecules in order of increasing boiling point.
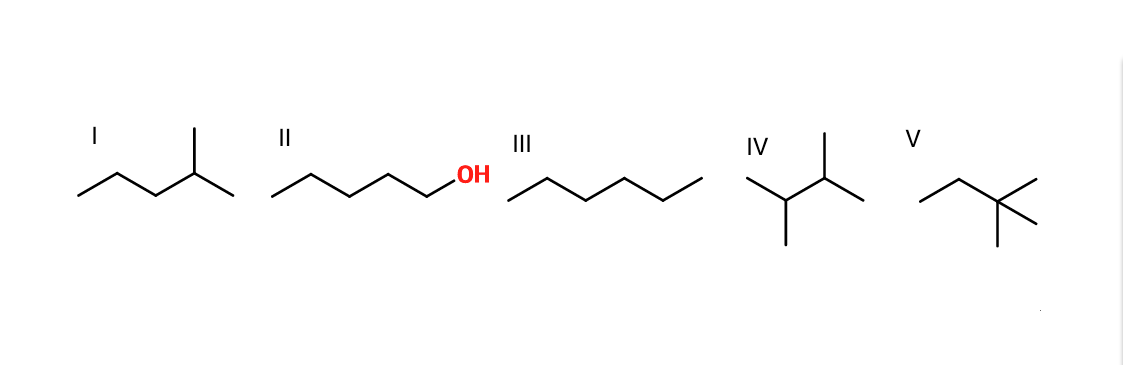
II, III, I, IV, V
IV, V, I, III, II
II, V, IV, I, III
I, IV, III, V, II
V, IV, I, III, II
V, IV, I, III, II
Most polar (II) has highest boiling point due to hydrogen bonds. The other molecules: increasing boiling point with decreased branching of the molecule (the more branched, the less surface area, and the lower the boiling point due to molecular stacking).
Example Question #232 : Organic Concepts
Which of the following intermolecular forces is the strongest?
All of these have approximately the same strength
Hydrogen bonding
London dispersion forces
Ion-dipole interactions
Dipole-dipole interactions
Hydrogen bonding
The strongest of those listed s hydrogen bonding. This type of intermolecular force is the attraction that occurs between hydrogen atoms and the lone pairs on atoms of oxygen, nitrogen and/or fluorine. Hydrogen bonds are the strongest while dispersion forces are the weakest. The strength of hydrogen bonds is responsible for properties of water such as high specific heat capacity, high surface tension, cohesion, high boiling point, and other.
Example Question #13 : Intermolecular Forces And Stability
A researcher is trying to identify a molecule. He observes that there is a weak hydrophobic bond between adjacent molecules. He also notices a weak polar interaction between the molecules. Which of the following could be the identity of the molecule?
Hexane
Hydrobromic acid
More than one of the above could be the identity of this molecule
Hydrochloric acid
More than one of the above could be the identity of this molecule
Intermolecular bonds occur between adjacent molecules (recall that ‘inter’ means ‘between’). There are several types of intermolecular bonds. Hydrophobic bonds, or van der Waals forces, are the weakest intermolecular forces and occur between every molecule; therefore, all of the listed molecules in the question have hydrophobic bonds. Polar interactions between molecules occur between charged species or polar molecules. Recall that polar molecules are molecules that contain two or more atoms with very different electronegativities. The more electronegative atom pulls the electrons closer to itself, causing polarity in the molecule. The more electronegative atom will have a partial negative charge (due to the proximity to electrons) and the less electronegative atom will have a partial positive charge. This polarity in molecule allows for dipole-dipole interactions, a type of intermolecular force.
To solve this question, we need to determine which molecules are polar. Hexane, or 


Example Question #231 : Organic Concepts
Which of the following is considered the strongest intermolecular bond?
Dipole-dipole interactions
Hydrogen bond
Ionic bond
Covalent bond
Hydrogen bond
Intermolecular bonds occur between adjacent molecules whereas intramolecular bonds occur within molecules. Examples of intermolecular bonds include hydrogen bonds, van der Waals interactions, and dipole-dipole interactions. The strongest intermolecular bond is hydrogen bond whereas the weakest is the van der Waals interactions. Covalent bonds and ionic bonds occur within molecules and are termed intramolecular bonds. Covalent bond is the strongest intramolecular bond.
Example Question #11 : Help With Intermolecular Forces
Which type of intermolecular force explains why butanal has a lower boiling point than octanal?
Ion-dipole interactions
Dipole-dipole interactions
Hydrogen bonding
van der Waals interactions
Covalent bonding
van der Waals interactions
As a molecule's mass increases, van der Waal forces also increases due to an increased area for fleeting charged interactions. Thus, the longer carbon chain length in octanal causes a higher boiling point. Because both octanal and butanal can participate in dipole-dipole interactions, this does not differentiate their boiling points, as it would if butane and butanal were compared. Both compounds participate in hydrogen bonding, which will account for their relatively high boiling points, but both molecules share the increased boiling point due to this type of intermolecular force.
Example Question #235 : Organic Concepts
Which of the following element(s) is/are not involved in hydrogen bonds?
I. Nitrogen
II. Oxygen
III. Chlorine
I and III
I only
I and II
III only
III only
Hydrogen bonds are strong intermolecular bonds between hydrogen and one of three atoms: nitrogen, oxygen and fluorine. A typical hydrogen bond occurs between a hydrogen atom on one molecule and one of the three atoms listed on another molecule. These bonds are reversible; however, they serve as strong interactions that stabilize a mixture of molecules.
Example Question #17 : Intermolecular Forces And Stability
It is observed that molecule A has a higher boiling point than molecule B. Which of the following could be the possible identities of molecule A and molecule B?
I. Molecule A: Hydrochloric acid, Molecule B: Hydrofluoric acid
II. Molecule A: Hydrogen peroxide, Molecule B: Diamond
III. Molecule A: Diamond, Molecule B: Nitric oxide
II and III
I and II
I only
II only
II only
Recall that boiling is the process of converting a liquid to a gas. This process involves the separation of molecules, which requires breaking the intermolecular bonds; therefore, boiling points depend on the strength of the intermolecular bonds. A stronger intermolecular bond will require more energy to break and, therefore, will have a higher boiling point. The question states that molecule A has the higher boiling point; therefore, molecule A must have stronger intermolecular interactions than molecule B. The strongest intermolecular bond is hydrogen bond, followed by dipole-dipole interactions and van der Waals interactions (weakest).
If we look at scenario I, molecule A is polar and has dipole-dipole interactions and van der Waals interactions (every molecule has van der Waals). It does not have hydrogen bonds because it does not contain nitrogen, oxygen, or fluorine (in addition the the hydrogen atom). Molecule B, on the other hand, has hydrogen bonds in addition to other intermolecular forces; therefore, molecule B has stronger intermolecular forces and a higher boiling point.
In scenario II, hydrogen peroxide has hydrogen bonds whereas diamond only has weak van der Waals interactions; therefore, molecule A has higher boiling point. In scenario III, diamond only has weak van der Waals interactions whereas nitric oxide can participate in dipole-dipole interactions as well (note that nitric oxide doesn’t have hydrogen atom and, therefore, cannot participate in hydrogen bonds). This means that molecule B has the higher boiling point in scenario III.
Example Question #233 : Organic Concepts
Which of the following compounds are not able to form hydrogen bonds with water?
Alkanes
Ethers
Carboxylic acids
Aldehydes
Alkanes
This question is rather straightforward, asking us which class of compounds will not form hydrogen bonds with water.
In order to form a hydrogen bond, whether it is intermolecular or intramolecular, there needs to be a partial positively charged hydrogen atom in between two other partial negative charged atoms. These atoms tend to be highly electronegative, and are usually either nitrogen, oxygen, or flourine.
Carboxylic acids will certainly engage in hydrogen bonds. The oxygen that is double bonded to the carbon has a partial negative charge, while the carbon has a partial positive charge, just as in aldehydes and ketones. Furthermore, the hydroxyl group attached to the carbon atom can also take part in hydrogen bonds.
Ethers are compounds in which an oxygen atom is situated between two carbon atoms via single bonds. Because there is a sufficient difference in the electronegativity of oxygen and carbon, ethers are also capable of hydrogen bonding.
Alkanes are hydrocarbons. This means that the only atoms found in these molecules are carbon and hydrogen. Because there is little difference in electronegativity between carbon and hydrogen, alkanes are incapable of hydrogen bonding with water.
Example Question #14 : Intermolecular Forces And Stability
Which of these accurately describes hydrogen bonds?
They are not involved in protein structure.
They decrease the boiling point of water.
They play an important role in the solvent properties of water.
They may occur between hydrogen and chlorine.
They play an important role in the solvent properties of water.
A hydrogen bond forms when a hydrogen attached to an electronegative atom of one molecule becomes attracted to an electronegative atom of another molecule (the electronegative atoms that may form hydrogen bonds are oxygen, nitrogen, and fluorine). Hydrogen bonds are extremely important in water molecules. The hydrogen atoms attached to the electronegative oxygen atom in water can form hydrogen bonds with the oxygen atoms of other water molecules, giving water many of its properties as a solvent.
Hydrogen bonds are also important in protein secondary structure, which is defined by the pattern of hydrogen bonds that form between the carbonyl oxygen and amine hydrogen atoms in the peptide backbone of proteins. Lastly, hydrogen bonds increase boiling point because they increase the strength of different substances.
Example Question #241 : Organic Concepts
Which of the following molecules would have the highest boiling point?
Boiling point increases as the strength of intermolecular forces of a substance increases. The strength of intermolecular forces of a substance increases with a longer carbon chain, branching of elements off of the carbon chain, and the addition of 






Certified Tutor
All Organic Chemistry Resources








