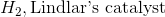All Organic Chemistry Resources
Example Questions
Example Question #552 : Organic Chemistry
Starting with an alkyne, synthesis of a cis alkene is driven upon addition of which of the following reagents?
Reduction of an alkyne with hydrogen and Lindlar's catalyst will result in a cis alkene. While 
Example Question #31 : Hydrocarbon Reactants

Predict the major product of the given reaction.
I
II
III
IV
V
IV
Hydrogen gas reacts with alkynes in the presence of a Lindlar catalyst by syn addition of hydrogen, resulting in a cis alkene product. A Lindlar catalyst is "poisoned" (often with lead) such that the reaction stops primarily at the alkene product. The correct answer is the option in which a cis-double bond is formed, compound IV.
Example Question #31 : Hydrocarbon Reactants
Which of the following would reduce 2-butyne into cis-2-butene?
Only 



Example Question #33 : Hydrocarbon Reactants
What is the major product of the given reaction?
1.
2.
3.
All are possible, depending acidic, basic, or neutral conditions
Here, we have a terminal alkyne reacting with what appears to be a very complicated and overwhelming set of reagents. This set of reagents, however, are simply used in the oxidation of pi bonds (in a reaction called hydroboration oxidation). In the process, 

If the reaction would have stopped here, answer choice 

Example Question #34 : Hydrocarbon Reactants
Which of the following sequences of reagents will produce the product from the starting material as shown below?




The formation of the halohydrin occurs when adding 
Example Question #32 : Hydrocarbon Reactants
What is the final product of the reaction shown?


I
IV
V
II
III
III
First step: elimination
Second step: Addition of two bromines across the double bond
Third step: Double dehydrohalogenation to form the alkyne, and removal of alkyne hydrogen (deprotonation)
Fourth step: methylation reaction (SN2)
Fifth step: metal-ammonia reduction forms trans alkene product
Example Question #36 : Hydrocarbon Reactants
What is the product of the reaction below?

The reaction shown is a metal-ammonia reduction of a terminal alkyne. This reduction will turn an alkyne into its corresponding trans alkene (however, since this alkyne is terminal, there is no cis/trans stereochemistry around the resulting alkene).
Note: the metal-ammonia reduction uses sodium metal and liquid ammonia (



Example Question #34 : Reactions By Reactant
What is the product of the reaction shown?
This reaction is how one would typically alkylate a terminal alkyne. The first step is to turn the alkyne into a good nucleophile. This is done by removing the hydrogen on the alkyne using sodium amide (

Certified Tutor
All Organic Chemistry Resources