All Organic Chemistry Resources
Example Questions
Example Question #1 : Hydrocarbon Products
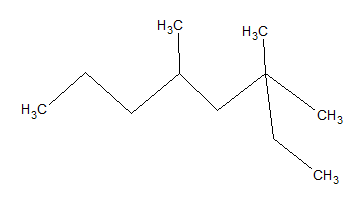
What is the IUPAC name of the given molecule?
3,3,5-trimethylnonane
3,3,5-trimethyloctane
None of these
2,2,4-trimethyloctane
4,6-dimethyl-6-ethylpentane
3,3,5-trimethyloctane
The longest carbon chain that can be formed is eight carbons. The base molecule is octane.
Using IUPAC rules, substituents should have the lowest possible numbers; thus, we start counting carbons from the right side rather than the left. If you count from the correct side, there are two methyl groups on carbon 3 and one on carbon 5. Thus, the name of the moleculue is 3,3,5-trimethyloctane.
Example Question #2 : Hydrocarbons

How could you brominate the compound?
None of these
Bromine gas
Bromine and UV light
Bromine and peroxides
Hydrobromic acid
Bromine and UV light
The given molecule is an alkane. The only way to brominate an alkane is with bromine gas and UV light. The energy from the light serves to creat two radical bromines. These radicals are capable of bonding with alkanes. If the given compound were an alkene, either hydrobromic acid or bromine and peroxides would work.
Example Question #1 : Reactions By Product
Predict the absolute configuration about the double bond formed in the given E1 reaction.

Racemic Z/E
No elimination reaction would proceed
E
Z
E
Unlike E2 reactions, in which hydrogen abstraction occurs simultaneously with the dissociation of the leaving group (limiting the configuration of the reaction's product), E1 reactions occur in two distinct steps. The slow rate-determining step that must first occur is the dissociation of the leaving group. Leaving behind a carbocation intermediate, it is often necessary to consider possible carbocation rearrangements that would stabilize the positive charge.
In this case, no such rearrangement is favorable as their are no locations of greater stability available.
However, what must be considered is that the intermediate is free to orient itself in its most stable conformation prior to the formation of the double bond in the second step. As a result, the E product (the larger substituents are on oriented opposite one another with respect to the double bond) is yielded primarily.
Example Question #2 : Reactions By Product
Which reagents are required to carry out the given reaction?
To carry out this reaction, we need to create a radical as an intermediate, which is an unpaired electron. We do so by introducing 

Certified Tutor
All Organic Chemistry Resources









