All MCAT Physical Resources
Example Questions
Example Question #1 : Vsepr Geometry
Which statement best describes VSEPR theory?
The repulsion between protons in an atom's nucleus determines its size
The repulsion between atoms helps determine the polarity of molecules
Covalent bonds are formed by overlapping valence electron shells
Molecular shapes are determined according to which orbitals are energetically accessible for bonding
The repulsion between electrons helps determine the geometry of covalent molecules
The repulsion between electrons helps determine the geometry of covalent molecules
The idea of VSEPR (valence shell electron pair repulsion) theory is that valence electron pairs repel each other, arranging themselves into positions that minimize their repulsions by maximizing the distance between them. The positions of these electron pairs then determine the overall geometry of the molecule. Molecular geometry is thus determined by the arrangement of electrons and nuclei such that the electrons are as far from one another as possible, while remaining as close to the positively charged nucleus as possible.
Example Question #861 : Mcat Physical Sciences
Which of the following compounds has a molecular tetrahedral geometry?
Of the available answer choices, only 



Example Question #2 : Vsepr Geometry
What is the molecular geometry of sulfur hexafluoride?
Square planar
Trigonal bipyramidal
Octahedral
Tetrahedral
Octahedral
Sulfur hexaflouride (SF6) is an example of octahedral geometry, as it follows the skeleton of AX6E0 format. A refers to sulfur, X to fluorine, and E to the lone pair electrons.
Square planar has an AX4E2 format, while tetrahedral and trigonal bipyramidal follow AX4 and AX5 formats, respectively.
Example Question #861 : Mcat Physical Sciences
Which of the following is not the correct geometric configuration for the given molecule?

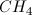




Recall the following relationships between geometry and number of pairs of electrons on the central atom.
2: linear
3: trigonal planar
4: tetrahedral
5: trigonal bipyriamidal
6: octahedral
To visualize the geometry, we need to think of how many electron pairs are on the central atom. Drawing Lewis dot diagrams may be helpful here. None of the answer choices has lone central electron pairs, with the exception of water, so the number of atoms bound to the central atom is the same as the number of central electron pairs.
The only one that does not match up with the correct geometry is SF6, which is actually octahedral since it has six central electron pairs. In a water molecule, the central oxygen has six valence electrons, plus one from each bond with hydrogen, for a total of eight central electrons and four central electron pairs. So, this geometry is a variation on the tetrahedral form (bent), in which two central electron pairs are not bound.
Example Question #862 : Mcat Physical Sciences
Which of the following molecules will have the smallest bond angles?
In order to determine which molecule will have the smallest bond angle(s), make sure to factor in both the number of atoms around the central atom as well any lone pairs on the central atom. 


Example Question #4 : Vsepr Geometry
The geometry of a certain molecule with the general formula 
Octahedral geometry always corresponds to the 








Example Question #863 : Mcat Physical Sciences
What is the geometry of 
Trigonal pyramidal
Square planar
Seesaw
Tetrahedral
Square pyramid
Tetrahedral
The central atom, sulfur, is surrounded by four electron groups (oxygen atoms), two of which are double bonded. Also note that the lone pairs are on the oxygen atoms, not the central atom. Thus the molecular geometry is tetrahedral.
Example Question #6 : Vsepr Geometry
According to the VSEPR theory, what is the angle between the two lone pairs in 
According to VSEPR theory, the electron pairs will repel each other as much as possible. Therefore, in the octahedral shape, the lone pairs will be on opposite ends of the molecule, or 
Certified Tutor
Certified Tutor
All MCAT Physical Resources




















