All MCAT Physical Resources
Example Questions
Example Question #691 : Mcat Physical Sciences
Hypothetically, if calcium hydroxide, lithium hydroxide, and potassium hydroxide all had a 

The solubilities of all three compounds are equal
Solubility is the ability of a solute to dissolve into a solvent. It is calculated by creating an equilibrium equation and solving for the concentration of dissolved ions.
For this question, we will need to set up equilibrium constant equations for each compound.
Note that the solid compounds are not included in the equilibrium expressions. Now we can work on finding the solubilities. For each mole of calcium hydroxide dissolved, one mole of calcium ions and two moles of hydroxide ions are released. For each mole of lithium hydroxide dissolved, one mole of lithium ions and one mole of hydroxide ions are released. For each mole of potassium hydroxide dissolved, one mole of potassium ions and one mole of hydroxide ions are released. Mathematically, we can equate these ratios to the ion concentrations in the equilibrium calculations.
In these calculations, 

Calcium hydroxide has the greatest solubility.
Example Question #11 : Solubility And Solution Equilibrium
The solubility product constant of 

Solubility is the ability of a solute to dissolve into a solvent. It is calculated by creating an equilibrium equation and solving for the concentration of dissolved ions.
For this question, we will need to set up the equilibrium constant equation for calcium hydroxide.
Note that the solid compound is not included in the equilibrium expression. Now we can work on finding the solubility. For each mole of calcium hydroxide dissolved, one mole of calcium ions and two moles of hydroxide ions are released. Mathematically, we can equate this ratio to the ion concentrations in the equilibrium calculation.
In this calculation, 

Example Question #21 : Solubility And Ions
How many moles of fluoride ions are present in 
Recall that the solubility product constant is given by the equation below, for a reaction in the following format.
Using our balanced reaction, we can find the solubility product equation for the dissociation of lead (II) fluoride.
For each molecule of lead (II) fluoride that dissolves, it produces one lead ion and two fluoride ions. We can conclude that the concentration of fluoride ions in solution will be twice the concentration of lead ions.
Use these variables in the solubility product equation, along with the given value from the question.
Now we can solve for the value of 
Remember that this value is equal to the concentration of lead ions in solution and half the concentration of fluoride ions in solution.
Example Question #21 : Solubility And Ions
Which of the following is true regarding the solubility product constant, 
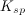
The concentration of substance 

As more of substance 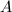

To determine the solubility product constant, we only need the concentrations and coefficients of the ions. The effective concentration of any pure substance (solid, liquid, or gas) is equal to one by definition, so does not influence the value of 
The units of the solubility product constant will depend on the coefficients of the products. 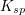
Example Question #22 : Solubility And Ions
Which of the following will not increase the solubility of a solution?
Increasing the temperature of a gas solute in a liquid solvent
Increasing the temperature of a solid solute in a liquid solvent
Decreasing the temperature of a gas solute in a liquid solvent
All of these will increase solubility
Increasing the pressure of a gas solute in a liquid solvent
Increasing the temperature of a gas solute in a liquid solvent
Increasing the temperature of a gas solute in a liquid solvent will decrease solubility. While dissolved, the gas is in equilibrium with the liquid. Adding heat will push the equilibrium in favor of the gas, causing it to precipitate from solution in the form of bubbles.
Increasing the pressure of a gas solute in a liquid solvent, decreasing the temperature of a gas solute in a liquid solvent, and increasing the temperature of a solid solute in a liquid solvent will all increase the solubility.
Example Question #22 : Solubility And Ions
Which of the following reactions will produce a precipitate?
Knowledge of basic solubility rules is enough to answer this question. Nitrates and salts of alkali metals are always soluble, as are salts of ammonium. Acetates are also always soluble. Acid-base reactions, such as with hydrofluoric acid, produce a soluble salt and water. Sulfates are commonly soluble, with certain exceptions (mostly alkaline earth metals).
The only reaction that produces an insoluble product is that between silver nitrate and potassium chloride. Silver chloride is a common precipitate obtained in double replacement reactions.
Example Question #1 : Solubility Rules
Boiling point is the temperature a liquid needs to achieve in order to begin its transformation into a gaseous state. Campers and hikers who prepare food during their trips have to account for differences in atmospheric pressure as they ascend in elevation. During the ascent, the decrease in atmospheric pressure changes the temperature at which water boils.
Further complicating the matter is the observation that addition of a solute to a pure liquid also changes the boiling point. Raoult’s Law can be used to understand the changes in boiling point if a non-volatile solute is present, as expressed here.
In this law, 


A scientist is studying boiling point changes with the addition of solutes, and creates a colloid. A colloid is similar to a solution because both will possess which of the following characteristics?
I. They both show the Tyndall effect on light
II. They both involve the suspension of only molecular-sized particles
III. They both involve the suspension of particles that are too small to be individually distinguished with the naked eye
I and II
III, only
I, II, and III
II and III
I and III
III, only
A colloid is a suspension of particles larger than molecules, but too small to be individually distinguished by the naked eye. Only colloids disperse light by the Tyndall effect. Milk is an example of a colloid.
Example Question #1 : Solubility Rules
Which of the following compounds would be generally insoluble in an aqueous solution?
MgCl2
NaOH
BaSO4
NH4OH
BaSO4
Knowing some of the general solubility trends will greatly facilitate your understanding of solubility in chemistry. Here are a couple guidelines you can follow in order to predict which compounds are soluble.
1. Compounds containing alkali metals, ammonium cations, or nitrate anions are soluble.
2. Compounds containing halogen anions are soluble. Key exceptions are halogens attached to silver, mercury, and lead.
3. Sulfates are soluble, except when attached to heavier alkaline metals, like barium (thus the correct answer).
4. Carbonates, phosphates, and hydroxides are generally insoluble; however, if they are attached to one of the ions mentioned in one of the above points, they are soluble.
Example Question #2 : Solubility Rules
Which of the following molecules is insoluble?
AgNO3
KBr
NaCl
AgCl
Mg(NO3)2
AgCl
These solubility rules should be known for the MCAT and are presented as a hierarchy.
1. All group 1 salts and ammonium salts are soluble
2. All nitrates, perchlorates, and acetates are soluble
3. All mercury, lead, and silver salts are NOT soluble
Understanding these solubility rules as a hierarchy, we can understand why Mg(NO3)2 and AgNO3 are soluble and why AgCl is not soluble.
Example Question #26 : Solubility And Ions
Which compound is most likely to be soluble in pentane?
Molecules are most soluble in solvents that possess similar properties and intermolecular forces to the solute.
Pentane is a non-polar compound, which interacts predominately through london dispersion forces. Ammonia, silver chloride, and phosphorus trichloride are all polar molecules. Ammonia has a lone pair on the nitrogen; silver is an ionic compound; phosphorus trichloride has lone pairs on both the phosphorus and chlorine atoms.
In contrast, carbon dioxide has lone pairs only on the oxygen atoms and has a linear geometry. This creates a line of symmetry in the molecule such that the net polarities of the carbon-oxygen bonds cancel each other. Even though carbon dioxide has polar bonds, it is not a polar molecule and would likely dissolve in pentane.
Certified Tutor
Certified Tutor
All MCAT Physical Resources





![Ca(OH)_2\rightleftharpoons Ca^{2+}+2OH^-;\ K_{sp}=[Ca^{2+}][OH^-]^2](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/113712/gif.latex)
![LiOH\rightleftharpoons Li^++OH^-;\ K_{sp}=[Li^+][OH^-]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/100505/gif.latex)
![KOH\rightleftharpoons K^++OH^-;\ K_{sp}=[K^+][OH^-]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/113713/gif.latex)











![Ca(OH)_2\rightleftharpoons Ca^{2+}+2OH^-;\ K_{sp}=[Ca^{2+}][OH^-]^2](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/100585/gif.latex)










![K_{sp}=[C]^c[D]^d](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/126068/gif.latex)

![K_{sp}=[Pb^{2+}][F^-]^2](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1053245/gif.latex)
![[Pb^{2+}] = x](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/102001/gif.latex)
![[F^-]=2x](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/126071/gif.latex)


![x = \sqrt[3]{\frac{3.7*10^{-8}}{4}}=2.1*10^{-3}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/126073/gif.latex)
![[Pb^{2+}]=x=2.1*10^{-3}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/102004/gif.latex)
![[F^-]=2x=2(2.1*10^{-3}M)=4.2*10^{-3}M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1051540/gif.latex)

![K_{sp}=[C]^c[D]^d](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/73718/gif.latex)
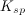
![K_{sp}=[C]^c[D]^d](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/100575/gif.latex)













