All MCAT Physical Resources
Example Questions
Example Question #11 : P H
You find a bottle in a lab that has a 
What is the pH of this solution?
Possible Answers:
Correct answer:
Explanation:

Alexandra
Certified Tutor
Certified Tutor
University of Central Florida, Bachelor of Science, Psychology. University of Central Florida, Master of Social Work, Social ...
All MCAT Physical Resources
Popular Subjects
Statistics Tutors in Atlanta, French Tutors in Philadelphia, ACT Tutors in New York City, Algebra Tutors in Chicago, GRE Tutors in Los Angeles, Chemistry Tutors in Boston, English Tutors in Los Angeles, Math Tutors in Miami, GMAT Tutors in Los Angeles, French Tutors in Denver
Popular Courses & Classes
SAT Courses & Classes in Miami, SSAT Courses & Classes in Houston, ACT Courses & Classes in New York City, SAT Courses & Classes in New York City, SSAT Courses & Classes in Phoenix, GRE Courses & Classes in Los Angeles, GRE Courses & Classes in Seattle, ACT Courses & Classes in Miami, ISEE Courses & Classes in Philadelphia, ACT Courses & Classes in Los Angeles
Popular Test Prep
GRE Test Prep in San Francisco-Bay Area, ACT Test Prep in Chicago, MCAT Test Prep in Phoenix, SAT Test Prep in Houston, MCAT Test Prep in Seattle, MCAT Test Prep in Dallas Fort Worth, GMAT Test Prep in Los Angeles, SAT Test Prep in Philadelphia, MCAT Test Prep in Denver, ISEE Test Prep in Denver





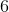

![[HNO_3]=[H^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/326585/gif.latex)
![pH=-log[H^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/326586/gif.latex)




