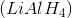All MCAT Biology Resources
Example Questions
Example Question #41 : Organic Chemistry, Biochemistry, And Metabolism
Consider the following radical bromination reaction of propane.
Which of the following substances would NOT be a suitable radical initiator for this reaction?

Azobisisobutyronitrile (or AIBN)
Benzoyl peroxide
Diisopropyl ether
UV light
tert-butyl peroxide
Diisopropyl ether
For any radical reaction, a suitable radical initiator is required. This either involves a chemical that decomposes to produce a radical upon heating, or ultraviolet light. For the former, any peroxide is suitable. AIBN also works, as it extrudes nitrogen upon heating to product a radical.
An ether, by contrast, will not produce a radical upon heating and would therefore not be a suitable radical initiator.
Example Question #42 : Organic Chemistry, Biochemistry, And Metabolism
Which of these is a dehydration synthesis reaction?
I. Formation of a disulfide bond between two cysteine residues
II. Carboxylation of a carbohydrate
III. Formation of an amide bond
IV. Splitting of dipeptides into amino acids
V. Addition of hydrogen to an unsaturated fatty acid
V
III
IV
I
II
III
Condensation reactions remove a small molecule—usually water—to bond two reactant molecules. In amide bonding, the carboxylic (–COOH, acid) portion of one amino acid is juxtaposed to the amide (–NH2, basic) end of a second amino acid. A molecule of water is extracted and the amide bond (–CONH) is formed. This is a variant of the classic statement, "Acid plus base yields salt plus water."
Technically, condensation reactions can remove small molecules other than water, but often the terms "condensation" and "dehydration" are used interchangeably in biochemistry (but not in organic chemistry). Incidentally, two cysteine molecules linked by a disulfide bond is renamed cystine (notice spelling alteration).
Example Question #11 : Reactions
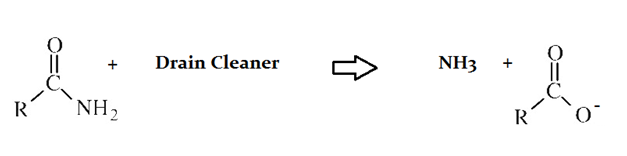
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
An alcohol reacts with the protein reactant in Reaction 2. Which atom in the protein reactant is likely to be the site of a nucleophilic attack?
The carbonyl oxygen and the nitrogen
The nitrogen
The carbonyl carbon
The carbonyl oxygen
Both the carbonyl carbon and the nitrogen
The carbonyl carbon
Carbonyl carbons bond with a strongly polar bond to the carbonyl oxygen. This strongly polar bond gives the carbon a partial positive charge, and thus makes it receptive to nucleophilic attack by other oxygens (e.g., alcohols).
Example Question #191 : Gre Subject Test: Chemistry
Which of these methods can be used to synthesize a secondary alcohol?
Reacting a ketone with lithium aluminum hydride, then adding water
All of these answer choices are correct
Adding a Grignard reagent to an aldehyde, then reacting with acid
Reacting propene with water and acid
All of these answer choices are correct
All of these methods can successfully synthesize secondary alcohols.
Lithium aluminum hydride and sodium borohydride are strong and weaker reducing agents, respectively. Both are able to reduce ketones to secondary alcohols.
Adding water and acid to an alkene (such as propene) results in Markovnikov addition of a hydroxyl group, also creating a secondary alcohol.
Grignard reagents (organometallic halides) add to the carbonyl carbon of an aldehyde, adding an alkane group and forming an alcohol product.
Example Question #2 : Other Reactions
Which of the following is an example of an anabolic reaction in the body?
Decomposition reactions
The conversion of glycogen to glucose
Hydrolysis
Condensation reactions
Condensation reactions
Anabolic reactions are reactions that construct molecules from smaller primary units. Catabolism is just the opposite, where larger molecules are degraded into simpler subunits. In the body larger macromolecules, such as proteins, are frequently created via condensation reactions. In condensation reactions, water is formed as a byproduct of joining two molecules together.
All other three processes describe a scenarios where a larger molecule is broken down into smaller subunits, known as catabolic processes.
Example Question #3 : Other Reactions
In the human body, glycerol and three fatty acids can act together to form triacyl glycerol in a process known as esterification. The reverse of this process is known as __________.
saponification
reverse esterification
transesterification
decarboxylation
saponification
When an alcohol and carboxylic acid are reacted together they can form an ester, in a process known as esterification. This is what happens with glycerol and fatty acids to form triacyl glycerol. The reverse of this process is an organic chemistry reaction known as saponification, in which an ester and water are reacted to form carboxylic acids and alcohol.
Example Question #43 : Organic Chemistry, Biochemistry, And Metabolism
Triacylglycerols are broken down in the body by lipases. In the presence of water, lipase is used to break down the triacylglycerol into a glycerol molecule and three fatty acids. Based on this information, the breakdown of a triacylglycerol is the opposite of which reaction?
Esterification
Hydrolysis
Decarboxylation
Saponification
Esterification
The molecular structure of a triacylglycerol reveals that the three fatty acid tails are attached to the glycerol molecule, resulting in three ester functional groups. Remember that fatty acids have a carboxylic acid functional group at one end, which is created after the cleavage described in the question. Since the ester bonds are broken and carboxylic acids are created following the hydrolysis of a triglyceride, the opposite of esterification has taken place.
The process described (breakdown of an ester into a carboxylic acid) is also known as saponification.
Example Question #44 : Organic Chemistry, Biochemistry, And Metabolism
Which of the following is not capable of oxidizing a secondary alcohol to a ketone?


Lithium aluminum hydride
All of these answers can oxidize secondary alcohols to ketones
Pyridinium chlorochromate (PCC)
Lithium aluminum hydride
Lithium aluminum hydride is correct because it is a reducing agent, and is therefore not capable of oxidizing secondary alcohols. Instead, LAH could be used to perform the reverse reaction, reducing a ketone to an alcohol. The other answer choices are oxidizing agents.
Example Question #49 : Organic Chemistry, Biochemistry, And Metabolism
Carbonic anhydrase is a very important enzyme that is utilized by the body. The enzyme catalyzes the following reaction:
A class of drugs that inhibits this enzyme is carbonic anhydrase inhibitors (eg. acetazolamide, brinzolamide, dorzolamide). These drugs are commonly prescribed in patients with glaucoma, hypertension, heart failure, high altitude sickness and for the treatment of basic drugs overdose.
In patients with hypertension, carbonic anhydrase inhibitors will prevent the reabsorption of sodium chloride 
When mountain climbing, the atmospheric pressure is lowered as the altitude increases. As a result of less oxygen into the lungs, ventilation increases. From the equation above, hyperventilation will result in more 
Carbonic anhydrase inhibitors are useful in patients with a drug overdose that is acidic. The lumen of the collecting tubule is nonpolar. Due to the lumen's characteristic, molecules that are also nonpolar and uncharged are able to cross the membrane and re-enter the circulatory system. Since carbonic anhydrase inhibitors alkalize the urine, acidic molecules stay in a charged state.
A patient overdosed on an unknown drug. Two hours after being admitted into the hospital, acetazolamide was administered. A realtime measurement of the drug's concentration in the patient's blood was taken the moment the patient was admitted every 30 minutes. Which of the following best explains the lab results shown?

The unknown drug was acidic
The unknown drug was basic
The unknown drug destroyed the renal system
The unknown drug was innate
The sample was influenced by the body's metabolic
The unknown drug was basic
From the data, the concentration of the unknown drug in the blood increased shortly after the patient received the carbonic anhydrase inhibitor. The increase in the blood's concentration of the drug meant that the body is reabsorbing the drug. The only way for the drug to be reabsorbed is if it is able to cross the membrane of the renal's lumen. In order to cross the renal's membrane, the drug must be noncharged and nonpolar. Basic molecules in a basic environment will stay uncharged.
Example Question #50 : Organic Chemistry, Biochemistry, And Metabolism
Carbonic anhydrase is a very important enzyme that is utilized by the body. The enzyme catalyzes the following reaction:
A class of drugs that inhibits this enzyme is carbonic anhydrase inhibitors (eg. acetazolamide, brinzolamide, dorzolamide). These drugs are commonly prescribed in patients with glaucoma, hypertension, heart failure, high altitude sickness and for the treatment of basic drugs overdose.
In patients with hypertension, carbonic anhydrase inhibitors will prevent the reabsorption of sodium chloride 
When mountain climbing, the atmospheric pressure is lowered as the altitude increases. As a result of less oxygen into the lungs, ventilation increases. From the equation above, hyperventilation will result in more 
Carbonic anhydrase inhibitors are useful in patients with a drug overdose that is acidic. The lumen of the collecting tubule is nonpolar. Due to the lumen's characteristic, molecules that are also nonpolar and uncharged are able to cross the membrane and re-enter the circulatory system. Since carbonic anhydrase inhibitors alkalize the urine, acidic molecules stay in a charged state.
Glaucoma involves the pressure within the eye increasing to a dangerously high level due to improper draining of the vitreous humor. Blindness usually follows due to damage to the nerves. How might administering a carbonic anhydrase inhibitor help in patients with glaucoma?
Increase intraocular pressure
Prevent the excess production of fluid
Increase the production of fluid
Increase acid production
Lower the rate of vitreous humor drainage
Prevent the excess production of fluid
From the equation:
We can see that carbonic anhydrase functions in both the forward and backward reactions. In the eye, the equation shifts to the left and more water is produced. Water is one of the main substances in vitreous humor. As the production of water increases, pressure increases as well. By blocking the production of water, the pressure is lowered.
Certified Tutor
Certified Tutor
All MCAT Biology Resources