All MCAT Biology Resources
Example Questions
Example Question #1581 : Mcat Biological Sciences
Which of these choices correctly pairs the level of protein structure with an example of that level of structure?
Primary structure is formed from alpha-helices
Tertiary structure is formed from disulfide bonds
Quaternary structure is formed from amino acids held together by peptide bonds
Tertiary structure is formed from beta-pleated sheets
Tertiary structure is formed from disulfide bonds
There are four distinct levels of protein structure: primary, secondary, tertiary, and quaternary. Primary structure refers to the actual sequence of amino acids, like Ala-Met-Gly-Trp, which are held together by peptide bonds. Secondary structure, which includes alpha-helices and beta-pleated sheets, is the local three-dimensional shape created by hydrogen bonding. Tertiary structure is the overall shape of the protein subunit, caused by more distant interactions. Disulfide bonds (bonds between the sulfur atoms of two cysteine amino acids) are an example of tertiary structure. Finally, quaternary structure involves interactions between the peptide subunits of a larger protein complex.
Example Question #2 : Protein Structure
Which level of protein structure is stabilized primarily by hydrogen bonding?
Quaternary structure
Hydrogen bonding does not significantly contribute to protein structure
Secondary structure
Primary structure
Tertiary structure
Secondary structure
Secondary structure is observed when the primary sequence of amino acids conforms into either alpha-helices and/or beta-pleated sheets. These conformations of the polypeptide chain are stabilized by hydrogen bonding alone.
Primary structure is determined by peptide bonds. Tertiary structure is determined by disulfide bonds and hydrophobic interactions. Quaternary structure is determined by interactions between multiple subunits.
Example Question #31 : Macromolecules
Hemoglobin is the principal oxygen-carrying protein in humans. It exists within erythrocytes, and binds up to four diatomic oxygen molecules simultaneously. Hemoglobin functions to maximize oxygen delivery to tissues, while simultaneously maximizing oxygen absorption in the lungs. Hemoglobin thus has a fundamentally contradictory set of goals. It must at once be opitimized to absorb oxygen, and to offload oxygen. Natural selection has overcome this apparent contradiction by making hemoglobin exquisitely sensitive to conditions in its microenvironment.
One way in which hemoglobin accomplishes its goals is through the phenomenon of cooperativity. Cooperativity refers to the ability of hemoglobin to change its oxygen binding behavior as a function of how many other oxygen atoms are bound to the molecule.
Fetal hemoglobin shows a similar pattern of cooperativity, but has unique binding characteristics relative to adult hemoglobin. Fetal hemoglobin reaches higher saturation at lower oxygen partial pressure.
Because of cooperativity, adult and fetal oxygen-hemoglobin dissociation curves appear as follows.

Beyond its ability to carry oxygen, hemoglobin is also effective as a blood buffer. The general reaction for the blood buffer system of hemoglobin is given below.
H+ + HbO2 ←→ H+Hb + O2
Because hemoglobin can act as a buffer in blood, it helps keep the pH constant. Which of the following portions of an amino acid can change with pH change?
Carboxy end
Side chain
Amino end and carboxy end
Amino end
Amino end, carboxy end, and side chain
Amino end, carboxy end, and side chain
All three portions can change with pH. The amino end can take on an extra proton to become positively charged, the carboxy end can lose a proton and take on a negative charge, and the side chain can do either depending on its structure. An amino acid with both a positively charged amino end and a negatively charged carboxy end is called a zwitterion.
Example Question #32 : Macromolecules
Collagen, the most abundant protein in the body, is an example of what type of protein?
Globular
Peripheral
Integral
Structural
Structural
Collagen is a structural protein that adds significant strength and resilience to the skin, tendons, and ligaments. Structural proteins, including collagen, also fall under the category of fibrous proteins. Globular proteins, in contrast, usually act as enzymes in the body or transport channels in the membrane.
Peripheral proteins are a type of globular protein found adjacent to the membrane, while integral proteins are transmembrane globular proteins.
Example Question #33 : Macromolecules
Amino acids are joined together to form polypeptides. Each amino acid is attached to another by a peptide bond.
What functional group is created when amino acids are joined together?
Ester
No new functional groups are created
Amide
Ketone
Amide
Polypeptide formation involves the C-terminus of one amino acid attaching to the N-terminus of another. This polymerization results in a dipeptide with the byproduct of one water molecule. The newfound combination results in a carbonyl being attached to a nitrogen. This functional group is called an amide.
Example Question #34 : Macromolecules
Hypersensitivity reactions occur when body tissues are affected by an abnormal immune reaction. The result is damage to normal tissues and clinical illness. A peanut allergy is an example of a hypersensitivity reaction, but there are three additional broad classes.
One class involves the abnormal production or deposition of antibodies. Antibodies are B-cell derived molecules that normally adhere to pathogens, rendering them unable to continue an infection. When antibodies are produced against normal tissues, however, disease can result. Figure 1 depicts a schematic structure of an antibody.
Antibodies can be divided into two peptide chains: heavy and light. Heavy chains form the backbone of the antibody, and are attached to light chains via covalent bonding. Each heavy and light chain is then further divided into constant and variable regions. Variable regions exhibit molecular variety, generating a unique chemical identity for each antibody. These unique patterns help guarantee that the body can produce antibodies to recognize many possible molecular patterns on invading pathogens.
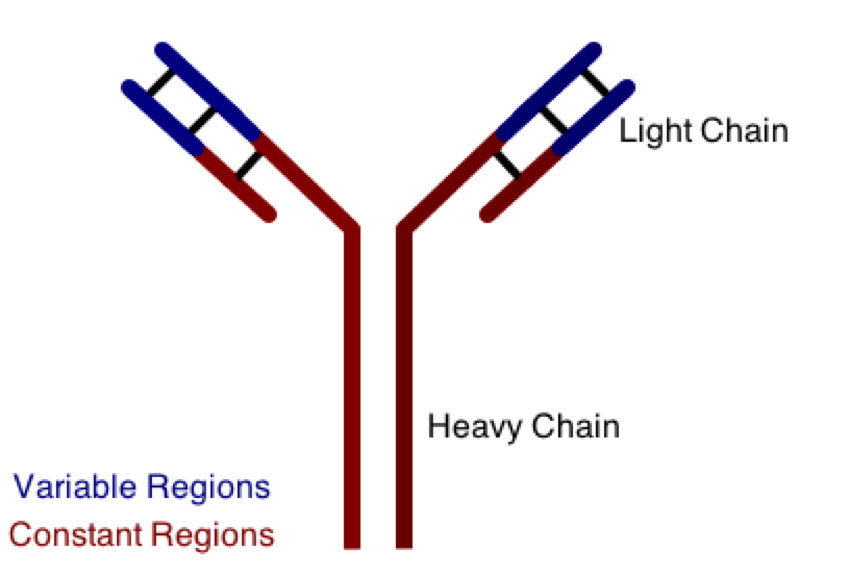
The polypeptides that make up the heavy and light chains of antibodies are most likely connected by covalent bridges involving atoms of which element?
Nitrogen
Sulfur
Carbon
Hydrogen
Oxygen
Sulfur
Covalent bridges can be found in organic molecules, linking one region of the molecule to another. These bridges are almost invariably disulfide linkages, in which two sulfur atoms form a covalent linkage that provides a great deal of stability between peptide chains. Disulfide bridges are commonly involved in protein tertiary structure and other organic structural linkages, such as the joining of the heavy and light chains in antibodies.
Example Question #35 : Macromolecules
Hypersensitivity reactions occur when body tissues are affected by an abnormal immune reaction. The result is damage to normal tissues and clinical illness. A peanut allergy is an example of a hypersensitivity reaction, but there are three additional broad classes.
One class involves the abnormal production or deposition of antibodies. Antibodies are B-cell derived molecules that normally adhere to pathogens, rendering them unable to continue an infection. When antibodies are produced against normal tissues, however, disease can result. Figure 1 depicts a schematic structure of an antibody.
Antibodies can be divided into two peptide chains: heavy and light. Heavy chains form the backbone of the antibody, and are attached to light chains via covalent bonding. Each heavy and light chain is then further divided into constant and variable regions. Variable regions exhibit molecular variety, generating a unique chemical identity for each antibody. These unique patterns help guarantee that the body can produce antibodies to recognize many possible molecular patterns on invading pathogens.

Antibodies are made of proteins, which form one of the broad classes of biological macromolecules. A glycoprotein is different from other kinds of proteins principally because __________.
glycoproteins are always lighter than other proteins
glycoproteins always act as hormonal messengers
glycoproteins always contain sugar moieties
glycoproteins are always heavier than other proteins
glycoproteins always interact with other proteins via van der Waals interactions
glycoproteins always contain sugar moieties
The prefix "glyco-" indicates that some substrate has had a carbohydrate moiety added to its structure. Glycolipids are thus lipids bound to saccharide units, and glycoproteins are proteins bound to saccharide units.
Example Question #36 : Macromolecules
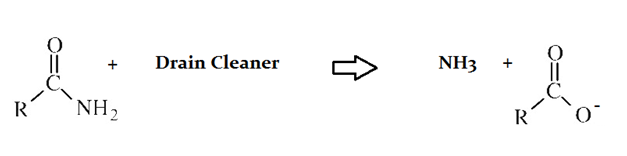
Drain cleaners are a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
In the proteins depicted in both reactions in the preceeding passage, which portions of the molecule are shown?
I. Amino terminus
II. Carboxy terminius
III. Side chain
II and III, only
I and III, only
I, only
III, only
II, only
I, only
The protein that is reacting with the drain cleaner in both instances shows the NH2 end, or amino terminus. The side chain and carboxy terminus are not shown.
Example Question #41 : Macromolecules
An enzyme that cleaves disulfide bridges would most disrupt a protein containing which amino acid sequence?
Val–Leu–Leu–Cys–Tyr–Thr
Cys–Leu–Val–Tyr–Tyr–Thr
All of the answers would be equally affected
Tyr–Cys–Cys–Thr–Val–Leu
Tyr–Cys–Val–Val–Leu–Thr
Tyr–Cys–Cys–Thr–Val–Leu
Disulfide bridges are made between two cysteine amino acids. An enzyme that cleaves disulfide bonds would disrupt a protein containing the most cysteine residues; therefore, Tyr–Cys–Cys–Thr–Val–Leu is the correct answer.
Example Question #1 : Cell Biology
Which of the following is an example of the secondary structure of a protein?
Hydrogen bonding between an amine and carbonyl group
Hydrogen bonding between R groups
Disulfide bonds between cysteine residues
Hydrophobic interactions
Peptide bonding between amino acids
Hydrogen bonding between an amine and carbonyl group
By definition, the secondary structure of a protein is the hydrogen bonding between the amine and carbonyl groups in the amino acid chain. This usually occurs in the form of alpha-helices or beta-pleated sheets.
The linear sequence of the amino acids formed by peptide bonds is the primary protein structure. Interactions of R groups determines the tertiary structure. These interactions can be in the form of disulfide bonds, hydrogen bonding, or hydrophobic interactions.
Certified Tutor
Certified Tutor
All MCAT Biology Resources