All MCAT Biology Resources
Example Questions
Example Question #1 : Functional Groups And Properties
Drain cleaners are a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
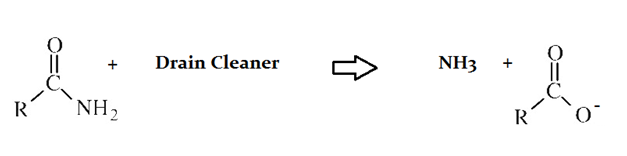
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
The NH4 molecule produced in Reaction 2 must have __________.
a basic nature due to the presence of the nitrogen atom
no charge, since N has a lone pair of electrons available to bond
a negative charge, since one was also created on the other product
a positive charge due to the presence of an extra hydrogen
an expanded octet for the N atom
a positive charge due to the presence of an extra hydrogen
The additional H present on ammonia (NH3) requires the generation of the a positive charge on the molecule. This also must be created to balance the negative charge created on the associated anion product.
Example Question #2 : Functional Groups And Properties
Which of the following amines is the strongest base?
A secondary amine with electron-withdrawing functional groups
A secondary amine with electron-donating functional groups
Ammonia
A primary amine with an electron-donating functional group
A secondary amine with electron-donating functional groups
Amines act as bases due to the lone pair on the nitrogen. Amine basicity can be increased or decreased by functional groups attached to the nitrogen. Electron-withdrawing groups decrease the basicity of an amine by lessening the effect of the lone pair, while electron-donating groups increase basicity by amplifying the lone pair effect. As a result, a secondary amine with an electron-donating functional groups will be the most basic amine.
Example Question #1741 : Mcat Biological Sciences
Ephedrine (shown below) contains what type of amine?

Secondary
Primary
Neutral
Quaternary
Tertiary
Secondary
A secondary amine is an amine (nitrogen atom) that is attached to two carbon-containing groups (alkyl groups or aryl groups). The nitrogen in ephedrine is attached to two alkyl groups, making it a secondary amine.
Primary amines are generally written as 

Example Question #2 : Functional Groups And Properties
Your lab isolates a compound with the formula 
Benzylamine
None of these
Benzylmethylamine
Nitrobenzene
Benzylamide
Benzylamine
Nitro groups and amide groups both contain oxygen components, and cannot be found in the compound described. We also know that the benzene ring only has a single constituent, meaning that it cannot be a methylamine. The compound must be benzylamine, a benzene ring with a -CH2NH2 substituent.
Example Question #3 : Functional Groups And Properties
What is the name of the functional group that contains a vinylic hydroxyl group?
Enol
Carboxyl
Keto
Aldehyde
Enol
Enol functional groups contain a hydroxyl group bonded to a carbon involved in a double bond with another carbon (vinyl carbon). An enol will usually undergo tautomerization to become a more stable keto.
Example Question #3 : Functional Groups And Properties
Ephedrine, whose structure is shown below, is used commonly as a stimulant and decongestant.

Ephedrine contains all of the following functional groups except __________.
alcohol
amine
arene
N-methyl group
ketone
ketone
Ephedrine contains an arene (the aromatic benzene ring), an alcohol (the -OH group), an amine (the nitrogen-based group), and an N-methyl group (-CH3 attached to nitrogen). It does not contain a ketone (C=O), or any other carboxyl groups.
Example Question #1 : Nucleophilic Addition
Which of the following compounds would you expect to undergo a nucleophilic addition reaction?
Ethanamide
Acetic acid
Methyl ethanoate
Propanal
Propanal
When dealing with carbonyl compounds, remember that a carboxylic acid and all of its derivatives will undergo nucleophilic substitution. Aldehydes and ketones will undergo nucleophilic addition. Propanal is a three-carbon aldehyde, and will thus undergo nucleophilic addition.
Acetic acid is a carboxylic acid, methyl ethanoate is an ether, and ethanamide is an amide; each of these would undergo nucleophilic substitution.
Example Question #5 : Functional Groups And Properties
Compound A, shown below, contains an example of what type of functional group?

Ketone
Ether
Ester
Carboxylic acid
Nitrile
Ester
Esters have the general molecular formula of 


Ketones have the formula of 



Compound A also contains an aromatic function group (the benzene ring) and a nitro group, 
Example Question #11 : Functional Groups And Properties
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
Which of the following would be expected pKa values for carbonic acid and carbonate, respectively?
14 and 1.4 * 10-14
1.4 * 10-14 and 14
10.3 and 6.3
pKa values depend on surrounding conditions
6.3 and 10.3
6.3 and 10.3
Carbonic acid is a weak organic acid, not nearly as strong as most inorganic acids. It is still, however, an acid, and has a pKa below 7, but nowhere near as low as 1.4 * 10-14.
Carbonate is a conjugate base, and thus has an alkaline pKa around 10.3.
Example Question #361 : Organic Chemistry, Biochemistry, And Metabolism
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
The deprotonation of carbonic acid is favored by __________.
resonance stabilization in carbon dioxide
a loss of CO2 from the system
resonance stabilization in carbonic acid
a buildup of bicarbonate in the system
resonance stabilization in bicarbonate
resonance stabilization in bicarbonate
Bicarbonate is the product of deprotonation of carbonic acid. Anything that stabilizes this product will encourage the deprotonation reaction, and resonance is a key stabilizing factor for bicarbonate. Stability of the conjugate base is a major contributing factor to the strength of an acid and its ability to deprotonate.
Certified Tutor
All MCAT Biology Resources