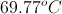All High School Physics Resources
Example Questions
Example Question #7 : Understanding Heat And Temperature
A sample of 



For this question, we must recognize that the system going to end up in equilibrium. That means that:
We are given the initial temperatures, masses, and specific heats of both the water and the ceramic. This will allow us to solve for the final temperature of the system; this value will be equal for both components. Notice that the specific heat given to us in the problem for the ceramic is in terms of kilograms, not grams. Convert to grams.
Example Question #8 : Understanding Heat And Temperature
A 



We need to know the freezing/boiling points of the liquid in order to solve.
We need to know the specific heat of the liquid in order to solve.
The equation for change in temperature is
Plug in our given values.
Notice that the specific heats will cancel out.
Combine like terms.
Example Question #611 : High School Physics
A 



The relationship between mass and temperature, when two masses are mixed together, is:
Using the given values for the mass and specific heat of each compound, we can solve for the final temperature.
We need to work to isolate the final temperature.
Distribute into the parenthesis using multiplication.
Combine like terms.
Example Question #1 : Understanding Effects Of Heat On Volume
An ideal gas is inside of a container with a pressure of 


We will need to use Boyle's Law to solve:
Boyle's Law allows us to set up a relationship between the changes in pressure and volume under conditions with constant temperature. Since the equation is a proportion, we do not need to convert any units.
We can use the given values to solve for the new pressure.
Example Question #1 : Understanding Effects Of Heat On Volume
An ideal gas is inside of a tube at 


For this problem, use Charles's Law:
In this formula, 

Using the given values, we should be able to solve for the final temperature.
Cross multiply.
Example Question #1 : Understanding Effects Of Heat On Volume
An ideal gas is compressed from 


For this problem, use Boyle's Law:
Boyle's Law allows us to set up a proportion between the pressure and volume at a constant temperature.
Using the values given, we can solve for the final pressure.
Example Question #612 : High School Physics
A balloon in a hot room is submerged in a bucket of cold water. What will happen to this balloon?
It will shrink
It will pop
It will lose air
It will not change
It will expand
It will shrink
The volume of air in the balloon will increase when exposed to hotter temperatures, and decrease when exposed to colder temperatures. If we look at the ideal gas law, we can see that temperature and volume have a direct relationship. As one goes down, so does the other, assuming all other factors remain constant.
We can also look at Charles's law of volumes:
The balloon is sealed, so the amount of gas in the balloon will not change, and the elasticity of the balloon means that pressure will also remain constant. As temperature decreases, volume must also decrease. Suppose that the temperature is halved in our question. The result would be half the volume, according to Charles's law.
By this logic, we can conclude that the balloon will shrink when placed in the cold water.
Example Question #611 : High School Physics
Why does adding heat cause a gas to expand?
Adding heat will not cause a gas to expand
Adding heat adds more molecules of gas to the system
Adding heat reduces the friction in the molecules when they are moving
Adding heat increases the velocity of the molecules
Adding heat causes the molecules to bond together, increasing their volume
Adding heat increases the velocity of the molecules
Heat is a form of energy. Adding heat to a gaseous system will increase the energy of the molecules, causing them to move faster and collide more frequently. This increased velocity results in the expansion of the gas.
Example Question #614 : High School Physics



The formula for the net energy added to a system is;
The change in energy equals the heat energy minus the work done.
We don't know the work, but we can solve for it using the work equation:
We are given both the force and the distance, allowing us to calculate the work.
Now that we know both the work done and the heat added, we can solve for the final energy of the system.
Example Question #612 : High School Physics
A certain amount of heat energy is added to a closed system. A few moments later, a scientist observes that the total increase in energy is LESS than that heat energy added to the system. Which could be a valid explanation for this conclusion?
Work was done by the system
The system is imperfectly designed
External forces are acting upon the system
The system is not actually closed
The measuring tools were incorrectly calibrated
Work was done by the system
The most likely explanation is that work is done by the system.
The formula for change in energy shows that the net change in energy is equal to the increase in heat energy minus the work done:
Since 
All High School Physics Resources