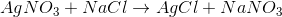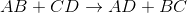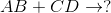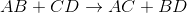All High School Chemistry Resources
Example Questions
Example Question #241 : High School Chemistry
What is the type of reaction seen below?
Addition
Decomposition
Single-replacement
Double-replacement
Double-replacement
By looking at the reaction, we see that the cations for the reactants have their anions switched in the products. This means that the reaction follows the general outline:
This is an example of a double-replacement reaction.
Addition reactions convert two reactants to a single product, while decomposition reactions convert a single reactant to multiple products. Single-replacement reactions only switch one cation-anion pair.
Example Question #1 : Help With Double Replacement Reactions
Predict the products for the following reaction if it were double displacement.
In a double displacement reaction, or double replacement reaction, opposite ions combine. What this means is that the cation of one compound will recombine and bond to the anion of the other compound and vice versa.
In this example, we have to remember the ideal convention of cations being written before the anion in the compound. So in AB, we can presume A being the cation and B being the anion. The same goes for CD - C is the cation and D is the anion.
With this in mind, we can now easily see how the replacement would be possible.
If A is a cation, and was originally bound to B (anion), the only other anion it is left with to bind is D. If C is a cation, and was originally bound to D (anion), it is only left with the option of binding to the now-free B anion.
That's why it is:
Example Question #31 : Chemical Reactions
What process could be used to describe the conversion of 

Radioactive decay
Reduction
Neutralization
Oxidation
Combustion
Reduction
The process illustrated is the reduction of the chromium atom. Reduction occurs when electrons are gained, causing the oxidation number (charge) of the atom to decrease. We can examine this process by looking at the oxidations numbers of each atom involved.
Oxygen always has an oxidation number of 

The initial oxidation state of chromium is 
Following the reaction, chromium has a charge of 

During the process, the chromium atom went from an oxidation state of 

Example Question #1 : Help With Oxidation Reduction Reactions
Determine which compound or ion is the oxidizing agent in the given reaction.
The oxidizing agent is the element that causes the other element to be oxidized, and is itself being reduced in the equation. Reduction is defined as the gain of electrons, meaning the element would get "more negative."
In this reaction, aluminum transitions from an oxidation state of 3+ to an oxidation state of 0. This indicates a reduction, as the atom has gained more electrons and reduced charge. 
Example Question #11 : Types Of Reactions
What is the oxidation number of sulfur in the given compound?
Use the rules for assigning oxidation numbers. We know that the sum of oxidation numbers of all atoms present in the compound must equal zero because the compound is neutral.
The oxidation number of hydrogen is 

The oxidation number of oxygen is always 

From here, simple math tells us that the oxidation number of sulfur must be 
Example Question #1 : Help With Oxidation Reduction Reactions
Which of the following describes a high energy, exothermic redox reaction that produces carbon dioxide and water?
Boiling
Combustion
Decomposition
Acid-base
Nuclear
Combustion
A combustion reaction is characterized as an oxidation-reduction reaction because the oxidation number of the reactants changes, and oxygen is used to burn a fuel molecule. Carbon dioxide is often produced as a product. The following is an example of a combustion reaction:

Example Question #242 : High School Chemistry
Which of the following describes an oxidation-reduction reaction?
None of these
A type of reaction in which one reactant is broken down into two products
A reaction in which one species molecule is oxidized then reduced
A type of chemical reaction that always involves acids and bases
The transfer of electrons between species, and the subsequent changes in oxidation states
The transfer of electrons between species, and the subsequent changes in oxidation states
In an oxidation reaction, one species is oxidized (loses electrons). During a reduction reaction, one species is reduced (gains electrons). These transfers of electrons results in the change of oxidation states. One way to help you remember which reaction is which, is by using the mnemonic: OIL RIG - Oxidation Is Loss of electrons; Reduction Is Gain of electrons.
Example Question #1 : Balancing Oxidation Reduction Reactions
What is the coefficient on sulfur dioxide if the following redox reaction is balanced in an acidic solution?
Balancing redox reactions involves the following steps:
1. Divide the reaction into oxidation and reduction half reactions and balance all atoms that are not oxygen and hydrogen:
2. Balance the oxygens by adding water molecules on the opposite side of the reactions:
3. Balance the hydrogens by adding protons to the opposite side of the equation:
4. Add electrons in order to equal the charges on both sides of the equation:
5. In order to make the electron exchange equal in each half step, we must multiply the top half reaction by 3:
6. Add up the reactants and products while cancelling out substances on opposite sides of the reactions. For example: we will cancel the 6 water molecules as reactants and be left with only one water molecule as a product. In addition, only 2 protons will be left on the reactant's side after canceling the 12 from the product's side.
In the balanced redox reaction, the coefficient on sulfur dioxide is 3.
Example Question #1 : Balancing Reactions
For the following unbalanced redox reaction, how many electrons are transferred and which chemical species is being oxidized?
One electron is transferred; Hg is oxidized
Two electrons are transferred; Hg is oxidized
One electron is transferred; P is oxidized
Two electrons are transferred; P is oxidized
Two electrons are transferred; Hg is oxidized
To begin, we will need to separate the given reaction into the two half-reactions by identifying changes in oxidation number. In this case, mercury (Hg) and phosphorus (P) show a change in oxidation number. Mercury begins with an oxidation number of zero, and ends with an oxidation number of 



Now we can begin to look at the half-reactions.
Balance the atoms.
Now balance the electrons. We know that each mercury atom loses one electron and each fluorine atom gains one electron.
We can see that two electrons are tranferred. To identify the element being oxidized, we must find the element that is losing electrons. In this case, mercury is being oxidized.
Example Question #241 : High School Chemistry
Oxidation is the __________ of electrons, reduction is the __________ of electrons.
loss . . . gain
gain . . . gain
gain . . . loss
loss . . . loss
None of these; it depends on the reaction.
loss . . . gain
An oxidation-reduction (redox) reaction is a reaction where electrons are transferred between two substances. When an atom is oxidized, it is called the reducing agent, and it loses a number of electrons. Similarly, a reduced atom is called the oxidizing agent, and gains the same number of electrons. A popular mnemonic to help remember is OIL RIG, or Oxidation is Loss of electrons, Reduction is Gain of electrons.
All High School Chemistry Resources