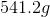All College Chemistry Resources
Example Questions
Example Question #1 : General Topics
Which of the following is neither a product nor a reactant in a combustion reaction?
Oxygen
All of these
Hydrocarbons
Water
Carbon Dioxide
All of these
A combustion reaction involves the release of heat from the burning of a hydrocarbon in oxygen to form carbon dioxide and water. The amount of hydrogen, carbon, and oxygen are conserved. This reaction is exothermic because of the high energy released when organic hydrocarbons are burned. It can be easily observed in the burning of petroleum distillates such as gasoline.
Example Question #2 : General Topics
Which type of reaction is shown?
Synthesis
Double displacement
Single displacement
Decomposition
Double displacement
There are five main types of chemical reactions.
1) Synthesis reactions have the following format:
In these reactions, two substances combine to form one substance. This occurs when hydrogen and oxygen combine to form water:
2) Decomposition reactions have the following format:
In these reactions, a compound breaks down to form smaller substances. This occurs when some acids decompose into an acidic oxide and water:
3) Single displacement reactions have the following format:
In these reactions, a single element replaces another element in a compound. This occurs in the reaction between zinc and hydrochloric acid:
4) Double displacement reactions have the following format:
In these reactions, an element from each of the two reactant compounds switches places. This occurs in the reaction between sulfuric acid and sodium hydroxide:
5) Combustion reactions have the following format:
In these reactions, a hydrocarbon and oxygen always react to form carbon dioxide and water. Here is an example:
Example Question #2 : General Topics
Identify the type of reaction that is given by the following equation:
Gas evolution
Precipitation
Acid-base
Oxidation-reduction
Oxidation-reduction
Recall that combustion equations are examples of reduction-oxidation reactions.
This cannot be a precipitation reaction because there are no solids in the products.
This cannot be a gas-evolution reaction because the reactants are not in aqueous form.
This cannot be an acid-base reaction because there is no proton transfer.
Example Question #4 : General Topics
What type of reaction is shown?
Decomposition
Double displacement/metathesis
Single displacement
Combination
Combustion
Combination
This is a basic identify the reaction type equation of the form:
Since both reactants are combined into one it is a combination reaction.
Now let's go over the wrong answers:
1) It isn't a single displacement because single displacement reactions are of the form:
2) It isn't a double displacement/metathesis reaction because double displacement reactions are of the form:
3) It isn't a decomposition reaction because decomposition reactions are the exact opposite of what occurs in the reaction in the problem. Meaning instead of two or more elements forming a compound, a compound breaks down into two or more elements.
4) It isn't a combustion reaction because a combustion reaction refers to the burning of a compound (usually a hydrocarbon) within which the other reactant is 

Example Question #1 : Reaction Chemistry
What is the balanced chemical equation for the combustion of butane 
Combustion is the chemical reaction of a hydrocarbon with molecular oxygen, and it always produces carbon dioxide and water. Knowing the reactants and products, the unbalanced equation must be:
We start by balancing the hydrogens. Since there are 10 on the left and only 2 on the right, we put a coefficient of 5 on water.
Similarly, we balance carbons by putting a 4 on the carbon dioxide.
To find the number of oxygens on the right, we multiply the 4 coefficient by the 2 subscript on O (which gets us 8 oxygens) and then add the 5 oxygens from the 5 water molecules to get a total of 13. The needed coefficient for 
Because fractional coefficients are not allowed, we mutiply every coefficient by 2 to find our final reaction:
Example Question #1 : Balancing Chemical Equations
When balanced, what is the value of 
Recall that a balanced chemical equation has the same number of each element on one side.
Start by counting the number of each element on each side.
There are the following numbers of moles of each reactant:
There are the following numbers of moles of each product:
Add coefficients in front of the molecular compounds in the equation until there are the same numbers of sulfur, oxygen, lithium, and selenium on each side.
The balanced chemical equation is the following:
Both products and reactants now have the following number of moles:
Since the coefficient in front of 



Example Question #2 : Balancing Chemical Equations
Balance the following chemical equation:
A balanced chemical equation will have the same number of each atom on both sides of the reaction.
Start by balancing the number of nitrate.
Since we have 

This equation now gives 

This equation is balanced because there are equal numbers of each atom on both sides.
Example Question #1 : Stoichiometric Calculations And Dimensional Analysis
Consider the unbalanced equation for the combustion of pentane:
How many grams of 


Start by balancing the given chemical equation:
Next, convert the grams of pentane into moles of pentane.
Use the stoichiometric ratio given by the balanced equation to find the number of moles of oxygen needed to react with the pentane.
Finally, convert the number of moles of oxygen into grams of oxygen.
There should be 

Example Question #2 : Stoichiometric Calculations And Dimensional Analysis
Consider the following unbalanced reaction:
How many grams of 




Start by balancing the equation:
Next, figure out which reactant is the limiting reactant.
Since fewer moles of 


Convert the amount of moles of 
Example Question #7 : General Topics
What is the mass in 


For this question, we're given the density and volume of a gas and we're asked to find the mass of the gas.
To answer this question, we'll need to use dimensional analysis. What this means is that we'll need to cancel out units in order to obtain the ones that we're looking for.
All College Chemistry Resources