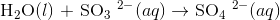All College Chemistry Resources
Example Questions
Example Question #11 : Reactions
In which of the following compounds is the oxidation state of phosphorus the least?
Let's start by examining each of the compounds we are given case-by-case and applying the general rules of oxidation numbers we know.
Let's start with 
We know that based off of the general rules of oxidation states, Oxygen generally has an oxidation number of 



Now we can solve for the oxidation state of phosphorus:
Since we have 



This is equal to 

Solving for 


Next let's looking at 
Knowing that an atom in its elemental form has an oxidation state of 

Now let's look at 
Knowing that the oxidation number of hydrogen is 


so 

Now let's consider 
Using our elementary rules for the oxidation numbers, we know oxygen has a 



Therefore our equation is:
therefore 

Finally let's look at 
Using the rules stated above for 
Therefore 

This means that the compound with the lowest oxidation state is 
Example Question #1 : Assigning Oxidation States
What is the oxidation state of gold in 
Let's first consider the overall compound 






We can now solve for oxidation state of gold through creating an equation as shown below:
Simplifying this we get that 
Example Question #7 : Assigning Oxidation States
What species is reduced in the following chemical reaction?
Reduction is defined as the gain of electrons so we want to find the element that gains electrons/becomes negatively charged.
Once again our equation is
Let's start on the reactant side with 
Following our general rules of oxidation states, we know that an atom in its elemental form has an oxidation state equal to zero, therefore 

Next, consider 



We can now solve for the oxidation state of 


Now let's consider 
Applying the rule for the oxidation number of oxygen being 
where 
This simplifies so that 

Moving on to the products, let's consider 
We know that the oxidation state of Oxygen is 


Now we must solve for sulfur's oxidation state:
therefore 

As for 


Looking at the changes of oxidation numbers between elements in the products and reactants we see that there is no change in the oxidation state of sulfur, hydrogen, and oxygen between the products and reactants, but lead goes from 

Example Question #2 : Assigning Oxidation States
What is the oxidation state of copper in 
In order to determine the oxidation state of copper in this compound we first must note the fact that compound has an overall charge of 
We can also apply the general rules of oxidation numbers we know to determine the oxidation numbers of hydrogen and oxygen. Since hydrogen is a group 



As for oxygen, based off of our general rules of oxidation numbers, we know that oxygen normally has an oxidation number of 
therefore 

Example Question #101 : College Chemistry
What is the oxidation number of sulfur in 
In order to determine the oxidation number we must apply the general rules of oxidation numbers we know. One of these rules states the oxidation number of fluorine is always 



This equation is 



therefore the oxidation number of sulfur is 
Example Question #11 : Reactions
Consider the reaction shown below.
Which of the following is true regarding this redox reaction?










For this question, we're given a reduction-oxidation (redox) reaction. We're asked to identify an answer choice that correctly states what the reducing agent is and what the oxidizing agent is.
Notice that another way we can write this reaction is by breaking up the ions as if they were dissociated in solution.
Notice in the above example that the potassium ion remains in the same oxidation state. This is what is known as a spectator ion, meaning that it doesn't really participate in the reaction and remains unchanged on reactant and product sides of the equation. Hence, we can remove it from both sides to simplify things.
In the simplified reaction shown above, we can see that the chlorine element is being reduced, while the iodine is being oxidized. Since the chlorine is being reduced, the iodine must be acting as the agent responsible for this. Hence, the 

Remember that the 

Example Question #12 : Reactions
Which compound is the reducing agent in this reaction?:
There is none.
A reducing agent is the compound in the reactants which becomes oxidized.
Oxidation is defined as the loss of electrons and is shown by the element changing to have a more positive oxidation number in the reaction.
To determine the reducing agent we simply see which compound is oxidized by determining the oxidation numbers of the elements in both the reactants and products, and comparing them.
Reactants:
For 
Same goes 
Products:





Results:
Since 
None of the products can be the reducing agent because this reaction isn't reversible.
Example Question #13 : Reactions
In this reaction, which compound is the reducing agent?:
This reaction isn't a redox reaction.
This reaction isn't a redox reaction.
The reducing agent is the compound which contains the element being oxidized. Oxidation is defined as the loss of electrons, so the element which is oxidized becomes more positively charged.
In order to determine which element is oxidized we need to calculate the oxidation numbers of all the elements in the reactants and products and compare them. The element which has an oxidation number that increases overall is the one that is oxidized. We determine the oxidation numbers by applying the elementary rules of oxidation numbers to each compound.
For reference the chemical equation is:
Let's start with the reactants:









Products:






Since there are no changes in the oxidation numbers of any of these elements, this isn't a redox reaction therefore there is no reducing agent.
Example Question #15 : Reactions
Which compound is the oxidizing agent?:
This isn't a redox reaction.
The oxidizing agent is the compound which contains the element being reduced. Reduction is defined as gaining of electrons, so the element which is reduced becomes more negatively charged.
In order to determine which element is reduced we need to calculate the oxidation numbers of all the elements in the reactants and products and compare them. The element which has an oxidation number that decreases overall is the one that is reduced and therefore the oxidizing agent. We determine the oxidation numbers by applying the elementary rules of oxidation numbers to each compound.
For reference the chemical equation is:
Let's start with the reactants:








In order to determine what phosphorus is we must look at the phosphate ion 








Products:









Now let's compare the oxidation numbers of each element:
Oxygen, phosphate, and sulfur have the same oxidation states in both products and reactants.
Chromium increases from an oxidation state of 

Finally, manganese decreases from an oxidation state of 


Example Question #11 : Redox Reactions
Balance the following redox reaction in acidic conditions:
Start by writing the half reactions for the equation
All atoms except for O and H are already balanced, so we can go straight to balancing O and H atoms. First balance O atoms by adding H2O
Now balance H atoms with H+
Balance each half reaction's electrical charge with electrons
Now combine both half reactions to get the overall reaction. Be sure to cancel out electrons by multiplying the oxidation reaction by 5 and the reduction reaction by 2. This will give 10 e- on each side of the overall reaction so that they cancel out.
Overall Reaction:
Notice that we have H2O and H+ on both sides of the equation, so we need to simplify. Subtract the 5 H2O on the reactant side from the 8 H2O on the product side which leaves 3 H2O on the reactant side. Then subtract the 10 H+ on the product side from the 16 H+ on the reactant side which leave 6 H+ on the reactant side. Now we have the simplified overall reaction
All College Chemistry Resources