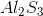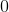All College Chemistry Resources
Example Questions
Example Question #1 : Ionic Bonds
In an ionic bond, electrons are __________.
repelled
excited
destroyed
shared
transferred
transferred
In an ionic bond, electrons are transferred to make a compound. For example, a sodium atom and a chloride atom can combine, exchanging electrons to form: 
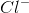

Example Question #1 : Ionic Bonds
Which of these can be formed when ionic bonds break down?
None of these; ionic bonds never break down.
Lattice structure
Micelle
Cation
Cation
Ionic bonds are bonds involving the attraction between oppositely-charged ions. An example of an ionic compound is 


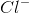
Ionic compounds have a characteristic lattice structure--that is, the arrangement of ions in a regular, geometric pattern. In the case of 



Lastly, micelles are lipid molecules which, in aqueous solution, arrange themselves in a spherical form. This is a response to the fact that fatty acids have both hydrophilic and hydrophobic regions (they are amphipathic). It has nothing to do with ionic bonds.
Example Question #1 : Molecules And Compounds
What is the molecular formula for aluminum sulfide?
Sulfur is much more electronegative than Aluminum (a metal), so when they bond they form an ionic compound as Aluminum transfers its electrons to Sulfur. Aluminum has a valence of 3, meaning that each Aluminum atom is looking to share or transfer 3 electrons to achieve a stable configuration. Sulfur has a valence of 6, meaning that each Sulfur atom is looking for two electrons to complete its octet. Thus, two Aluminum atoms will bond with three Sulfur atoms, resulting in the transfer of six electrons in total.
Example Question #3 : Ionic Bonds
What is the charge on the cation in the ionic compound sodium oxide?
When sodium and oxygen form the ionic compound sodium oxide, 
All College Chemistry Resources