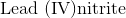All College Chemistry Resources
Example Questions
Example Question #6 : Molecular Geometries And Vsepr Theory
What is the molecular geometry of the molecule 
Trigonal pyramidal
Trigonal planar
Square pyramidal
T-shaped
Trigonal bipyramidal
T-shaped




Example Question #32 : Molecules And Compounds
Which of the following is true of both molecular orbitals and atomic orbitals?
The number of molecular orbitals is equal to the number of atomic orbitals for a given molecule.
The number of molecular orbitals are half the number of atomic orbitals for a given molecule.
The number of atomic orbitals are twice the number of molecular orbitals for a given molecule.
Molecular orbitals are to valence bond theory as atomic orbitals are to atomic orbital theory.
No comparison between molecular orbitals and atomic orbitals can be made as they are consistent with different theories of bonding.
The number of molecular orbitals is equal to the number of atomic orbitals for a given molecule.
The number of molecular orbitals is equal to the number of atomic orbitals for a given molecule. The number of orbitals is consistent. Molecular orbitals shows destructive and constructive interference between sigma and pi bonds. In molecular orbital diagrams, these results appear as either bonding or anti bonding energies. Atomic orbitals combine the valence shells of overlapping orbitals to form more stable electron configurations.
Example Question #33 : Molecules And Compounds
What is the hybridization of a 
sp4
sp2
sp3d
sp
sp3d2
sp3d
The correct hybridization of a 



Example Question #32 : Molecules And Compounds
Name the compound:
First, recognize that this is an ionic compound.
Identify the cation and the anion. The cation is lead, and the anion is nitrate.
Since lead can form more than one type of ion, figure out the charge associated with the given cation.




The name of this compound is then 
Example Question #34 : Molecules And Compounds
What is the correct name for 
Chlorous acid
Hydrochloric acid
Chloric acid
None of these
Perchloric acid
Perchloric acid






Example Question #35 : Molecules And Compounds
Consider the following the trend of 




Hypobromic acid
Bromous acid
Hydorbromic acid
Perbromic acid
Hypobromous acid
Perbromic acid
Because there are four oxygen atoms like in perchloric acid, the correct name is perbromic acid. Hypobromic acid is not a valid name for an acid since the "hypo-" prefix refers to one or two oxygen atoms but the "-ic" suffix refers to either three or four oxygen atoms. Hypobromous and bromous acid refer to one and two oxygen atoms, respectively. Hydrobromic acid has no oxygen atoms.
Example Question #36 : Molecules And Compounds
What is the correct nomenclature for 
Sulfurous acid
Sulfuric acid
Hydrogen sulfide
Hydrogen sulfite
Hydrogen sulfate
Sulfurous acid
The correct nomenclature for 




Example Question #4 : Nomenclature And Functional Groups
What is the correct molecular formula for cobalt (II) sulfite?
The correct molecular formula for cobalt (II) sulfite is 



Example Question #32 : Molecules And Compounds
What is the oxidation state of manganese (

When assigning oxidation states to elements of a given compound, non-transition metal elements are assigned specific oxidation states corresponding to their group number and valence relative to a complete octet.
Group 1 elements have 1 valence electron and an oxidation state of +1.
Group 2 elements have 2 valence electrons and an oxidation state of +2.
Group 8 elements (the noble gases) have a complete octet, thus their assigned oxidation state is 0.
Group 7 elements (halogens) have 7 valence electrons and an oxidation state of -1.
Group 6 elements such as oxygen have 6 valence electrons and an oxidation state of -2.
Permanganate has 4 oxygen atoms and an overall charge of -1. The oxidation state of the 

and finding the difference between their combined oxidation state and the overall charge of the ion (-1):
Example Question #33 : Molecules And Compounds
According to VSEPR theory, for the ammonium ion (
tetrahedral . . . trigonal pyramidal
trigonal pyramidal . . . tetrahedral
trigonal pyramidal . . . trigonal pyramidal
tetrahedral . . . tetrahedral
tetrahedral . . . tetrahedral
Both the electron-pair and molecular geometries of ammonium are tetrahedral, as opposed to ammonia (
Ammonia:

Note that the difference in geometry arises in the presence of the lone pair on nitrogen in ammonia. The lone pair only contributes to molecular geometry, but does not contribute to electron-pair geometry. Bonding electrons, however, contribute to both electron and molecular geometries.
Ammonium:

In ammonium, the lone pair seen in ammonia is shared in a bond with an additional hydrogen atom.
Certified Tutor
Certified Tutor
All College Chemistry Resources