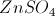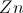All AP Chemistry Resources
Example Questions
Example Question #1 : Principles Of Oxidation Reduction Reactions
Consider the following reaction:
Which of the following statements is true?
Zinc is oxidized and copper is reduced
Zinc is reduced and copper is oxidized
Sulfur is reduced and copper is reduced
Sulfur is oxidized and copper is reduced
Zinc is oxidized and copper is reduced
For any oxidation-reduction reaction, the oxidized atom will lose electrons and the reduced atom will gain electrons. Zinc is originally in elemental form, so it has an initial oxidation state of zero. As a product, it has a charge of 
Copper originally has an oxidation state of 
Sulfur remains in the sulfate complex ion, which retains its overall 
Example Question #1 : Principles Of Oxidation Reduction Reactions
In this reaction, electrons are transferred from ___________ to ___________.
hydrogen . . . calcium
calcium . . . hydrogen
calcium . . . chlorine
hydrogen . . . chlorine
chlorine . . . hydrogen
calcium . . . hydrogen
A transfer of electrons indicates an oxidation-reduction reaction. The element losing electrons is oxidized, while the element gaining electrons is reduced.
Calcium is initially a neutral solid, but as a product has a charge of 
Hydrogen initially has a charge of 
We can thus conclude that calcium was oxidized and hydrogen was reduced, indicating that electrons were transferred from calcium to hydrogen.
The charge on chlorine remains 
Example Question #1 : Principles Of Oxidation Reduction Reactions
In the following reaction, which compound is being oxidized?
Copper
No oxidation takes place
Nitrate
Sulfur
Hydrogen
Sulfur
Hydrogen doesn't change. Cu2+ doesn't change (partnered with S2- then with SO42-). Sulfur goes from S2- and S6+(paired with 6 O2- with a 2– charge), showing an oxidation. Nitrogen goes from N5+ to N2+ meaning it was reduced.
Example Question #2 : Principles Of Oxidation Reduction Reactions
Consider the following reaction:
What is the oxidizing agent, and what is the reducing agent?
Silver is the oxidizing agent and there is no reducing agent
Silver is the oxidizing agent and copper is the reducing agent
No redox chemistry occurs
Silver is the reducing agent and there is no oxidizing agent
Silver is the reducing agent and copper is the oxidizing agent
Silver is the oxidizing agent and copper is the reducing agent
Let's break down the reaction into two separate reactions:

We can see that copper loses electrons, while silver gains electrons. Recall that oxidation is loss and reduction is gain, with regard to electrons. Copper is oxidized and silver is reduced.
However, this question asks for the oxidizing agent and reducing agent. Recall that the oxidizing agent is reduced, while the reducing agent is oxidized. Since copper is oxidized, it is the reducing agent. Similarly, since silver is reduced, it is the oxidizing agent.
Example Question #133 : Reactions And Equilibrium
What does oxidation mean?
Gain of electrons
Loss of neutrons
Loss of electrons
Gain of protons
Loss of protons
Loss of electrons
Think "OIL RIG" Oxidation Is Loss of electrons, Reduction Is Gain of electrons. The loss and gain of electrons is what changes the charge of the atom or molecule. In redox reactions, electrons are transferred. Changing the number of protons changes the identity of the element ,which is not the case. Since chemical reactions involve valence electrons, the nucleus is unchanged.
Example Question #7 : Principles Of Oxidation Reduction Reactions
Which statement about the reaction equation is correct?

This is a hydrolysis reaction

This is a combustion reaction






Example Question #8 : Principles Of Oxidation Reduction Reactions
Oomycetes of the genus Phytophthora are capable of causing economic damage by destroying crops. The name Phytopthora from the Greek phyton = plant, and phthora = destruction literally means plant-destroyer. However, chemists may battle their destructive effects.
The compound named copper(II) carbonate can be used to destroy the plant-destroyer, and can be produced as shown in the following equation:
What is the net ionic equation for the given reaction that produces copper(II) carbonate?
An ionic equation is a chemical equation in which ionic compounds in an aqueous solution are written as dissociated ions.
A complete ionic equation shows all the ions present in the solution (which includes both the reactive and spectator ions), as the dissociated ions. The complete ionic equation for this problem's reaction is:
It is worth noting that in a complete ionic equation, it becomes readily apparent which ions are involved in the oxidation-reduction reaction, and which ions are the spectator ions (those that do not change during the reaction). For example since 




The net ionic equation shows only the reactive ions in solution and the product(s) formed by them. Therefore a net ionic equation can most simply be made by looking at the complete ionic equation and omitting the spectator ions as shown below:
Net ionic equation:
Example Question #131 : Reactions And Equilibrium
Consider the following reaction:
Which of the following substances is the oxidizing agent?
In an oxidation-reduction reaction, the oxidizing agent is the reactant that accepts electrons, becoming reduced. The oxidizing or reducing agent is always the whole reagent, and is never just the atom in the reagent that is being oxidized or reduced.




Example Question #51 : Chemical Equilibrium
Calculate the equilibrium constant for 



Example Question #52 : Chemical Equilibrium
Calculate the equilibrium constant for 


73.2
25.3
50.5
0.143
15.8
50.5


Certified Tutor
All AP Chemistry Resources