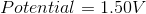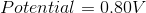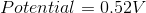All AP Chemistry Resources
Example Questions
Example Question #301 : Ap Chemistry
Consider the following balanced equation:
What is the difference between the oxidation state of aluminum on the right side of the equation versus the left?
No difference
On the left side of the equation, 
Sulfate, 
The difference comes from simple subtraction: 
Example Question #2 : Oxidation State
What is the oxidation number of Cr, S, and Fe in the following substances: (a) K2Cr2O7 (b) H2SO4 (c) Fe2O3.
3, 6, 3
3, 6, 6
6, 6, 3
3, 3, 3
6, 6, 6
6, 6, 3
(a) Since O has a –2 oxidation number and K has a +1 oxidation number (1 valence
electron it gives up), that means that Cr must have an oxidation number of +6. (b) Since H
has a +1 oxidation number and O has a –2 oxidation number, S has a +6 oxidation number.
(c) Fe has an oxidation number of +3 in order for it to have a net 0 oxidation state.
Example Question #2 : Oxidation State
In the above reaction, what are the initial and final oxidation states of 
There is no change in oxidation state
To determine the initial oxidation state of 









On the product side of the reaction, notice that 

Example Question #15 : Oxidation Reduction Reactions
The Claus process is used in the petroleum industry to convert sulfur containing gases into solid (rhombic) sulfur. One of the reactions that takes place in this process is:
Which of the following statements is correct:

This is a red-ox reaction

All of them are correct
All of them are correct
We have an oxidizing 





In total 32 electrons are transferred from the 

Example Question #1 : Reduction Potential
The standard reduction potentials for certain metals are listed below:
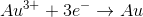




What is the standard potential of a cell in which copper is reduced by iron?
In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.

Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.

Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.

Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.
Example Question #311 : Ap Chemistry
The standard reduction potentials for some metals are listed below:





Which of the following metals is the strongest reducing agent?
The answer cannot be determined
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
Example Question #2 : Reduction Potential
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
–1.08 V
–0.40 V
0.46 V
1.08 V
–1.76 V
–1.08 V
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
Example Question #1 : Principles Of Oxidation Reduction Reactions
Methane combusts in the presence of oxygen according to the following reaction:
Which of the following statements is true concerning the reaction?
Oxygen is oxidized in the reaction
Carbon is oxidized in the reaction

Oxygen has a charge of 
Carbon has an initial oxidation state of
Carbon is oxidized in the reaction
By comparing the oxidation number of an atom as a reactant and its oxidation number as a product, we can determine if the atom has been oxidized or reduced. In increase in oxidation number indicates a loss of electrons, or oxidation. A decrease in oxidation number signals a gain of electrons, or reduction.
For electrochemistry, you should familiarize yourself with the traditional oxidation states of hydrogen 



Carbon is initially in the form of methane, meaning that it is attached to four hydrogen atoms. The molecule is neutral, and each hydrogen has an oxidation number of 

In the a product, carbon is attached to two oxygens, each with a charge of 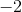

Since carbon went from an oxidation state of 
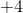
Example Question #2 : Principles Of Oxidation Reduction Reactions
Which of the following reactions involves the transfer of electrons?
Decomposition
Redox
Neutralization
Combination
Redox
Redox reactions occur when one species is oxidized—loses electrons—and another is reduced—gains electrons. Thus, this is the only one of the given options that involves the transfer of electrons.
Example Question #1 : Principles Of Oxidation Reduction Reactions
Which statement about this reaction is FALSE?

Chromium is oxidized during the reaction
Aluminum is oxidized during the reaction.
Chromium is the oxidizing reagent for this reaction.
This reaction is an example of a single displacement reaction.
Chromium is oxidized during the reaction
In Cr2O3, Cr has a charge of +3. The chromium metal product is neutral, and therefore has a charge of 0. So, Cr is reduced from +3 to 0.
Remember, OIL RIG. Oxidation Is Loss (of electrons) and Reduction Is Gain (of electrons).
Certified Tutor
All AP Chemistry Resources