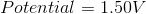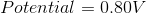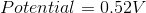All AP Chemistry Resources
Example Questions
Example Question #61 : Reaction Types
When is the oxidation number of H (-1)?
We typically think of Hydrogen as having an oxidation number of +1. However when it is bonded to a less electronegative element such as Na it is actually assigned an oxidation number of -1.
Example Question #1 : Oxidation State
What is the oxidation number of manganese in 
Potassium always has an oxidation state of 


The most important rule of oxidation numbers is that their sum must equal the charge on the molecule. In this compound, we have 
Manganese needs to cancel out the charge from potassium and oxygen in order to give us a neutral compound.
Example Question #1 : Oxidation State
What is the oxidation number of chlorine in perchlorate?
Perchlorate is a complex ion with the formula 
The most important rule for oxidation number is that the sum of the oxidation states of the atoms must equal the overall molecular charge.
This compound has four oxygens, which always have a 
Chlorine usually has an oxidation state of 
Chlorine must be 
Example Question #301 : Ap Chemistry
Consider the following balanced equation:
What is the difference between the oxidation state of aluminum on the right side of the equation versus the left?
No difference
On the left side of the equation, 
Sulfate, 
The difference comes from simple subtraction: 
Example Question #2 : Oxidation State
What is the oxidation number of Cr, S, and Fe in the following substances: (a) K2Cr2O7 (b) H2SO4 (c) Fe2O3.
3, 6, 3
3, 6, 6
6, 6, 3
3, 3, 3
6, 6, 6
6, 6, 3
(a) Since O has a –2 oxidation number and K has a +1 oxidation number (1 valence
electron it gives up), that means that Cr must have an oxidation number of +6. (b) Since H
has a +1 oxidation number and O has a –2 oxidation number, S has a +6 oxidation number.
(c) Fe has an oxidation number of +3 in order for it to have a net 0 oxidation state.
Example Question #2 : Oxidation State
In the above reaction, what are the initial and final oxidation states of 
There is no change in oxidation state
To determine the initial oxidation state of 









On the product side of the reaction, notice that 

Example Question #15 : Oxidation Reduction Reactions
The Claus process is used in the petroleum industry to convert sulfur containing gases into solid (rhombic) sulfur. One of the reactions that takes place in this process is:
Which of the following statements is correct:

This is a red-ox reaction

All of them are correct
All of them are correct
We have an oxidizing 





In total 32 electrons are transferred from the 

Example Question #1 : Reduction Potential
The standard reduction potentials for certain metals are listed below:
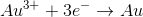




What is the standard potential of a cell in which copper is reduced by iron?
In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.

Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.

Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.

Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.
Example Question #311 : Ap Chemistry
The standard reduction potentials for some metals are listed below:





Which of the following metals is the strongest reducing agent?
The answer cannot be determined
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
Example Question #2 : Reduction Potential
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
–1.08 V
–0.40 V
0.46 V
1.08 V
–1.76 V
–1.08 V
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
Certified Tutor
All AP Chemistry Resources