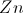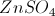All AP Chemistry Resources
Example Questions
Example Question #81 : Reaction Types
Consider the following reaction:
Which of the following substances is the oxidizing agent?
Possible Answers:
Correct answer:
Explanation:
In an oxidation-reduction reaction, the oxidizing agent is the reactant that accepts electrons, becoming reduced. The oxidizing or reducing agent is always the whole reagent, and is never just the atom in the reagent that is being oxidized or reduced.




Kaustabh
Certified Tutor
Certified Tutor
New York University, Bachelor of Science, Chemistry. Stanford University, Doctor of Philosophy, Chemistry.
All AP Chemistry Resources
Popular Subjects
SSAT Tutors in San Diego, Math Tutors in Phoenix, ACT Tutors in San Diego, LSAT Tutors in Chicago, Math Tutors in Seattle, French Tutors in Atlanta, Math Tutors in San Diego, Chemistry Tutors in Los Angeles, Algebra Tutors in Dallas Fort Worth, LSAT Tutors in Atlanta
Popular Courses & Classes
Spanish Courses & Classes in Los Angeles, ISEE Courses & Classes in Washington DC, GMAT Courses & Classes in San Diego, SSAT Courses & Classes in San Francisco-Bay Area, ISEE Courses & Classes in Denver, MCAT Courses & Classes in Dallas Fort Worth, GMAT Courses & Classes in Dallas Fort Worth, LSAT Courses & Classes in Los Angeles, GRE Courses & Classes in Philadelphia, GRE Courses & Classes in Los Angeles
Popular Test Prep
GRE Test Prep in San Francisco-Bay Area, SSAT Test Prep in Washington DC, GRE Test Prep in Philadelphia, ISEE Test Prep in Denver, ISEE Test Prep in Los Angeles, ACT Test Prep in Seattle, ISEE Test Prep in Washington DC, ISEE Test Prep in New York City, SAT Test Prep in San Francisco-Bay Area, SAT Test Prep in Los Angeles