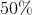All AP Chemistry Resources
Example Questions
Example Question #31 : Calculations
Calculate and give the answer with the correct number of significant figures.
For multistep calculations, make sure to keep track of the number of significant figures at the end of each step so we know how many significant figures to round to at the end of the entire calculation. To maintain accuracy, do not round intermediate steps.
First, do the two multiplication problems found within the parentheses.
Recall that for multiplication, the result needs to have the same number of significant figures as the factor with the least number of significant figures. Our answer from the addition should then only have 3 significant figures since that is the number of significant figures 3.14 has. To remember that the answer should only have 3 significant figures, the last significant digit will be highlighted:
Recall that for multiplication, the result needs to have the same number of significant figures as the factor with the least number of significant figures. Our answer from the addition should then only have 1 significant figure since that is the number of significant figures 10 has. To remember that the answer should only have 1 significant figure, the last significant digit will be highlighted:
Now, multiply:
Since the factor with the least number of significant figures only has 1 significant figure, the answer must also be rounded to 1 significant figure. 5570.13393 is rounded to 6000.
Example Question #31 : Calculations
Calculate and give the answer to the correct number of significant figures.
For multistep calculations, make sure to keep track of the number of significant figures at the end of each step so we know how many significant figures to round to at the end of the entire calculation. To maintain accuracy, do not round intermediate steps.
First, do the two multiplication problems found within the parentheses.
Recall that for multiplication, the result needs to have the same number of significant figures as the factor with the least number of significant figures. Our answer from the addition should then only have 1 significant figure since that is the number of significant figures 0.02 has. To remember that the answer should only have 3 significant figures, the last significant digit will be highlighted:
Next, do the addition.
Because this is addition, the result of the calculation needs to have the same number of decimal places as the number with the fewest decimal places. We need to only keep 1 decimal place because of 
Next, do the second addition.
Because this is addition, the result of the calculation needs to have the same number of decimal places as the number with the fewest decimal places. Since 10 does not have any decimal places, our final answer also cannot have any decimal places. 33.356 is rounded down to 33.
Example Question #31 : Laboratory Techniques And Analysis
3 mols of 


What is the percent conversion of 
To find the percent conversion, use the following equation:
Initially there are 3 mols of 



Plugging this into the above equation gives a conversion rate of 58%.
Example Question #211 : Ap Chemistry
You run an experiment in order to empirically find the gas constant, 
You know from the literature that
What is the percent error in your experimental value of 
To find the percent error in your 
Doing this, we find the percent error to be 4.6%.
Example Question #35 : Calculations
Suppose that a chemist working in a lab is trying to synthesize caffeine. In doing so, he predicts that he can produce 

When chemists synthesize compounds in the lab, a theoretical yield can be calculated by using knowledge of the full, balanced chemical equation, as well as the starting amounts of all reagents including the limiting reagent. However, due to a variety of factors, the actual yield obtained at the end of the experiment is nearly always going to be lower than the theoretical yield. Some of the things that can cause this are experimental error and side reactions, among others. Thus, chemists usually take the ratio of actual yield to theoretical yield to give the percent yield.
This means that the overall process was 
Example Question #31 : Laboratory Techniques And Analysis
During lab you have a solution of 1M NaCl at room temperature. You accidentally add so much salt to the solution that you still see some at the bottom of the beaker no matter how vigorously you mix it. What conclusion can you arrive at based upon your knowledge of chemistry?
The sodium chloride that sank to the bottom is a special isotope that can't be solubilized.
The saturation limit of the water for sodium chloride has been surpassed. This is a unsaturated solution.
The salt you spilled into the solution wasn't sodium chloride, but was magnesium chloride, which isn't soluble in water.
The saturation limit of the water for sodium chloride has been surpassed. This is a saturated solution.
The large spill caused hydrophobic clusters of sodium chloride to form, preventing them from being dissolved.
The saturation limit of the water for sodium chloride has been surpassed. This is a saturated solution.
The addition of so much salt (NaCl) caused the water to reach its solubility limit for NaCl. This means that no matter how much more NaCl you add to that solution or how hard you stir, it will not dissolve, and will precipitate on the bottom of the beaker. This type of solution is saturated. If the solution were unsaturated, then more salt would still be able to dissolve.
There is no special isotope that makes NaCl insoluble in water. The vigorous mixing would have separated any clumps, and if the solution was unsaturated the salt would have eventually dissolved. Magnesium chloride, another salt, is very soluble in water and would not result in the scenario described.
Example Question #1 : Laboratory Equipment And Procedure
There is a beaker full of a clear liquid on the counter. It was left unlabeled by your lab partner, and you are required to find out what it is. You perform a number of tests and observe the follwing results.
1. The liquid is miscible with water.
2. You add the liquid to 1M phosphoric acid. The beaker gets extremely warm.
3. You grab a piece of red litmus, and it turns blue upon exposure to the unknown liquid.
Which of the following could be the identity of the unknown liquid?
Each test gives us a different piece of information. The first test tells us that our compound is soluble in water. The second test tells us that heat is released when acid is added. The third test tells us that the solution turns red litmus paper blue, which means that the solution is basic. From these results, we can conclude that the compound is a water-soluble base. Now we can look at our answer options.





Example Question #1 : Laboratory Equipment And Procedure
Using the target shown, determine whether the shots were accurate and/or precise.
Precise but not accurate
Neither precise nor accurate
Precise and accurate
Accurate but not precise
Precise but not accurate
The target is precise because the shots are closely grouped together. However, the shots are not accurate because they are not in the middle of the target.
Example Question #1 : Laboratory Equipment And Procedure
Using the target, determine whether the shots are accurate and/or precise.
Accurate and precise
Neither accurate nor precise
Accurate but not precise
Precise but not accurate
Accurate and precise
The target is accurate because the shots are all in the middle of the target. It is also precise because all the shots are grouped together.
Example Question #1 : Drawing Conclusions From Observations
Using the target shown, determine whether the shots are accurate and/or precise.
Accurate but not precise
Accurate and precise
Precise but not accurate
Neither accurate nor precise
Accurate but not precise
The shots are not precise because they are not grouped together. However, one of the shots is in the center of the target, and thus is accurate.
Certified Tutor
Certified Tutor
All AP Chemistry Resources