All AP Chemistry Resources
Example Questions
Example Question #31 : Acid Base Chemistry
A 1M solution of a monoprotic acid has a pH of 4.6. What is the 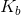
In order to find the base dissociation constant for the conjugate base, we can start by finding the acid dissociation constant for the acid. Since a 1M solution of the acid has a pH of 4.6, we can find the proton concentration of the solution.
Since the acid is monoprotic, we can set the following equilibrium expression equal to its acid dissociation constant.
We can see that, since the acid is monoprotic, the concntration of protons will be equal to the concentration of the acid anion. The final concentration of the acid molecule will be equal to the initial concentration, minus the amount of protons formed. Using these values, we can solve for the equilibrium constant for the acid.
Now that we have the acid dissociation constant, we can find the conjugate base's dissociation constant by setting the product of the two values equal to the autoionization of water.
Example Question #181 : Reactions And Equilibrium
Would H2SO4 or HNO3 produce a more acidic solution?
H2SO4 since it has a lower pKa
H2SO4 since it has a higher pKa
HNO3 since it has a lower pKa
HNO3 since it has a higher pKa
H2SO4 since it has a lower pKa
Both are strong acids, but H2SO4 is bivalent, realeasing 2 protons for each molecule dissolved in solution. Further, a more acidic solution would have a lower pKa.
Example Question #121 : Reaction Types
Which of the following species will not be present in an aqueous solution of 
All of these will be present in the solution
The hydroxide ion (OH–) is a strong base and therefore would not be present in an acidic solution. Protons, the hydronium ion, and water will all be present in relatively large amounts within the solution.
Example Question #982 : Ap Chemistry
Which of the following anions is the strongest base?
Weaker acids dissociate less in water and therefore, reverse reaction is favored in




Example Question #81 : Thermodynamics
Consider the following electrolytic cell:

What happens at the anode in the electrolytic cell?
Nickel is reduced
Nickel is oxidized
Iron is reduced
Iron is oxidized
Nickel is oxidized
It does not matter if the cell is galvanic or electrolytic; oxidation will always take place at the anode. This means that the nickel loses two electrons and is oxidized at the anode to generate nickel ions.
Nickel ions and iron are products, and are neither oxidized nor reduced during the reaction. Iron ions are reduced at the cathode to generate the iron product.
Example Question #981 : Ap Chemistry
Consider the following reaction in a galvanic cell:
Which of the following takes place at the anode?
Zinc is oxidized
No reaction takes place at the anode
Copper is reduced
Zinc is reduced
Copper is oxidized
Zinc is oxidized
Oxidation takes place at the anode and reduction takes place at the cathode. You can remember this with the pneumonic "An Ox, Red Cat."
In the equation, zinc loses electrons. It goes from a neutral, elemental charge to a charge of 
Example Question #145 : Thermochemistry And Kinetics
Toward which pole do electrons travel in a galvanic cell?
Towards the cathode
Towards the negative pole
More information is needed
Electrons do not travel; only the protons travel
Towards the anode
Towards the cathode
Reduction always occurs at the cathode, and oxidation always occurs at the anode. Since reduction is the addition of electrons, the electrons must flow toward the site of reduction.
In a galvanic cell the positive charge is on the cathode, while the negative charge is on the anode. Since a galvanic cell has a positive potential and is spontaneous, electrons freely flow down their potential gradient. The electrons, which are negatively charged, are traveling towards the cathode, which is positive charged, since opposites attract.
Example Question #542 : High School Chemistry
Toward which pole do the electrons travel in an electrolytic cell?
Towards the cathode
Towards the anode
The electrons do not travel; only the protons travel
Towards the positive pole
More information is needed
Towards the cathode
Reduction always occurs at the cathode, and oxidation always occurs at the anode. Since reduction is the addition of electrons, electrons must travel toward the site of reduction.
In an electrolytic cell the negative charge is on the cathode, while the positive charge is on the anode. Since an electrolytic cell requires energy to perpetuate the reaction, we are pushing the electrons against their potential gradient. The electrons, which are negatively charged, are traveling towards the cathode, which is also negatively charged.
Example Question #1 : Electrochemistry
Which of the following differences between galvanic cells and electrolytic cells is false?
Electrolytic cells have a positive Gibb's free energy
Electrolytic cells have negative voltages
Electrolytic cells are non-spontaneous
Electrolytic cells have oxidation take place at the cathode
Electrolytic cells have oxidation take place at the cathode
Electrolytic cells use non-spontaneous reactions that require an external power source in order to proceed. The values between galvanic and electrolytic cells are opposite of one another. Galvanic cells have positive voltage potentials, while electrolytic voltage potentials are negative. Both types of cell, however, have oxidation occur at the cathode and reduction occur at the anode.
Example Question #1 : Galvanic (Voltai) And Elecrolytic Cells
What is true about the current flow in a galvanic cell?
It travels from cathode to anode
It flows in the direction of electron flow
Galvanic cells do not have a current
It travels from anode to cathode
It travels from cathode to anode
In a galvanic cell, the electrons will flow from the anode to the cathode; however, current travels in the opposite direction of the electrons by convention. In chemical cells, current is defined by the direction in which protons would flow. As a result, current flows from the cathode to the anode.
Keep in mind that reduction occurs at the cathode and oxidation occurs at the anode. Electrons are thus constantly moving from the anode to the cathode. Since the current will be opposite to the flow of electrons, we can conclude that it must flow from cathode to anode.
Certified Tutor
Certified Tutor
All AP Chemistry Resources



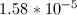

![pH=-\log[H^+]\rightarrow [H^+]=10^{-pH}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/121717/gif.latex)
![\small [H^{+}] = 10^{-4.6} = 2.51*10^{-5}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/94835/gif.latex)
![\small K_{a} = \frac{[H^{+}][A^{-}]}{[HA]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/121718/gif.latex)
![K_a= \frac{[2.51*10^{-5}]^{2}}{[1-2.51*10^{-5}]} = 6.31*10^{-10}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/94836/gif.latex)