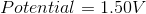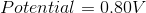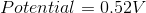All AP Chemistry Resources
Example Questions
Example Question #2 : Oxidation State
What is the oxidation number of Cr, S, and Fe in the following substances: (a) K2Cr2O7 (b) H2SO4 (c) Fe2O3.
3, 3, 3
3, 6, 3
3, 6, 6
6, 6, 3
6, 6, 6
6, 6, 3
(a) Since O has a –2 oxidation number and K has a +1 oxidation number (1 valence
electron it gives up), that means that Cr must have an oxidation number of +6. (b) Since H
has a +1 oxidation number and O has a –2 oxidation number, S has a +6 oxidation number.
(c) Fe has an oxidation number of +3 in order for it to have a net 0 oxidation state.
Example Question #12 : Oxidation Reduction Reactions
In the above reaction, what are the initial and final oxidation states of 
There is no change in oxidation state
To determine the initial oxidation state of 









On the product side of the reaction, notice that 

Example Question #17 : Oxidation Reduction Reactions
The Claus process is used in the petroleum industry to convert sulfur containing gases into solid (rhombic) sulfur. One of the reactions that takes place in this process is:
Which of the following statements is correct:
This is a red-ox reaction
All of them are correct


All of them are correct
We have an oxidizing 





In total 32 electrons are transferred from the 

Example Question #71 : Reaction Types
The standard reduction potentials for certain metals are listed below:
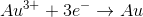




What is the standard potential of a cell in which copper is reduced by iron?
In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.

Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.

Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.

Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.
Example Question #311 : Ap Chemistry
The standard reduction potentials for some metals are listed below:





Which of the following metals is the strongest reducing agent?
The answer cannot be determined
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
Example Question #312 : Ap Chemistry
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
–0.40 V
–1.08 V
0.46 V
1.08 V
–1.76 V
–1.08 V
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
Example Question #1 : Solutions And States Of Matter
A mixture of neon gas and argon gas is present in a container (container A). There are equal amounts of both gases in the container. A small pinhole is created in the container, allowing the gases to effuse into an empty container (container B). The effusion time is very brief, and the pinhole is eventually plugged, resulting in a mixture of both gases in both containers.
Which of the following statements is true after the pinhole is plugged?
Container B will contain twice as many argon atoms as neon atoms
Container B will contain twice as many neon atoms as argon atoms
The partial pressure for argon is greater than the partial pressure for neon in container A
Both gases will have equal partial pressures in container A
The partial pressure for argon is greater than the partial pressure for neon in container A
The rate of effusion for two gases can be compared to one another using the following equation:
Here, the effusion rates are inversely proportional to the square root of the molecular masses of the gases in question. Because the relationship is to the square roots of the molecular masses, we will not observe a 2:1 ratio of effusion for neon compared to argon.
We will, however, see that more neon effuses out of container A compared to the amount of argon because neon is the lighter gas and will thus have a faster effusion rate. As a result, there will be more argon than neon in container A after the pinhole is plugged. This results in argon having a larger partial pressure than neon in container A.
Example Question #1 : Effusion
A glass box holds equal amounts of hydrogen, nitrogen, oxygen, and bromine. The gases are allowed to exit the container through a tiny hole. Which gas will exit the hole the fastest?
Nitrogen
Bromine
They all exit at the same rate because the temperature is constant
Oxygen
Hydrogen
Hydrogen
At a particular temperature, the average kinetic energy of all gaseous molecules is equal. Since hydrogen gas has the lowest mass out of these gases, it will have the highest average velocity. This means that it will exit out of the tiny hole at a rate faster than the other gases. Conversely, bromine, which has the most mass compared to the other gases, will exit the hole the slowest.
This relationship is mathematically represented in Graham's law:
As the mass increases, the rate of effusion decreases.
Example Question #1 : Effusion
Which of the following gases will have the highest rate of effusion?
Helium
Nitrogen
Oxygen
Carbon dioxide
Sulfur dioxide
Helium
The rate of effusion for a gas is inversely proportional to the square-root of its molecular mass (Graham's Law).
The gas with the lowest molecular weight will effuse the fastest.
Oxygen:
Nitrogen:
Carbon dioxide:
Sulfur dioxide:
Helium:
The lightest, and therefore fastest, gas is helium.
Example Question #1 : Solutions And States Of Matter
Molecule A has twice the mass of molecule B. A sample of each molecule is released into separate, identical containers. Which compound will have a higher rate of diffusion?
There is not enough information to determine relative rates of diffusion
Molecule A would have a faster initial rate; both molecules would reach an equal final rate
They will have identical rates of diffusion
Molecule B
Molecule A
Molecule B
According to Graham's law, the rate of diffusion of a gas molecule is inversely proportional to the root square of that molecule's mass. Because molecule B has a smaller mass than molecule A, it will have a higher rate of diffusion.
Note: this also applies to finding the rate of effusion.
All AP Chemistry Resources