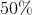All AP Chemistry Resources
Example Questions
Example Question #201 : Ap Chemistry
Calculate and give the answer using the correct number of significant figures.
First, do the calculation.
Because this is addition, the result of the calculation needs to have the same number of decimal places as the number with the fewest decimal places. Since 3 has the least number of places after the decimal point, the answer must not have any decimal points. The answer will round up to 25.
Example Question #202 : Ap Chemistry
Calculate and give the answer with the correct number of significant figures.
First, do the calculation.
Recall that for multiplication and division, the answer uses the least number of significant figures found in the question. In this case, 3.0 has the least number of significant figures at 2. The final answer should then be rounded up to only have 2 significant figures also. Thus we round up to 270.
Example Question #201 : Ap Chemistry
Calculate and give the answer using the correct number of significant figures.
For multistep calculations, make sure to keep track of the number of significant figures at the end of each step so we know how many significant figures to round to at the end of the entire calculation. To maintain accuracy, do not round intermediate steps. Do the addition and subtraction in the parentheses first.
Recall that for addition, the result needs to have the same number of digits after the decimal point as the number with the fewest decimal points in the question. Our answer from the addition should then only have 4 significant figures. To remember that the answer should only have 4 significant figures, the last significant digit will be highlighted:
Next, do the subtraction.
Since the rules for significant figures for addition and subtraction are the same, our answer here should only have 2 significant figures. The last significant figure from this step will also be highlighted: 
Now, divide these two numbers:
Now, this is when the highlighting of significant digits in earlier steps becomes important. Recall that for multiplication and division, the result has the same number of significant figures as the factor with the least number of significant figures.
Round the final answer to 2 significant figures to reflect the least amount of significant figures found in the division. Thus 43.40167364 rounds down to 43.
Example Question #203 : Ap Chemistry
Calculate and give the answer using the correct number of significant figures.
First, carry out the calculation.
Recall that for multiplication and division, the answer uses the least number of significant figures found in the question. In this case, 3.45 has the least number of significant figures at 3. The final answer should then be rounded up to only have 3 significant figures. 2023.720183 rounded to three significant figures is 2020.
Example Question #10 : Significant Figures
Calculate and give the answer using the correct number of significant figures.
For multistep calculations, make sure to keep track of the number of significant figures at the end of each step so we know how many significant figures to round to at the end of the entire calculation. To maintain accuracy, do not round intermediate steps. First, calculate what's found inside the parentheses.
Recall that for multiplication and division, the answer uses the least number of significant figures found in the question. In this case, 5 has the least number of significant figures at 1. The final answer should then be rounded up to only have 1 significant figure. The last significant digit will be highlighted:
Next, do the addition.
Because this is addition, the result of the calculation needs to have the same number of decimal places as the number with the fewest decimal places. Since 20 does not have any decimal places, our answer also cannot have any decimal places. Thus, 27.82 rounded to two significant figures is 28.
Example Question #21 : Laboratory Techniques And Analysis
Calculate and give the answer with the correct number of significant figures.
For multistep calculations, make sure to keep track of the number of significant figures at the end of each step so we know how many significant figures to round to at the end of the entire calculation. To maintain accuracy, do not round intermediate steps.
First, calculate what's found inside the parentheses.
Recall that for multiplication and division, the answer uses the least number of significant figures found in the question. In this case, both factors have 2 significant figures. The final answer should only have 2 significant figures also. The last significant digit will be highlighted: 
Next, do the addition.
Because this is addition, the result of the calculation needs to have the same number of decimal places as the number with the fewest decimal places. Since 20. does not have any decimal places, our answer also cannot have any decimal places. Thus 36.23 is rounded down to 36.
Example Question #22 : Calculations
Calculate and give the answer with the correct number of significant figures.
For multistep calculations, make sure to keep track of the number of significant figures at the end of each step so we know how many significant figures to round to at the end of the entire calculation. To maintain accuracy, do not round intermediate steps.
First, do the two addition problems found within the parentheses.
Recall that for addition, the result needs to have the same number of digits after the decimal point as the number with the fewest decimal points in the question. Our answer from the addition should then only have 2 significant figures. To remember that the answer should only have 2 significant figures, the last significant digit will be highlighted:
Perform the second addition using the same logic as above. To remember that the answer should only have 2 significant figures, the last significant digit will be highlighted:
Now, multiply:
Because both factors only have 2 significant figures, our final answer should also only have 2 significant figures. 0.33216 is rounded to 0.33.
Example Question #31 : Calculations
Calculate and give the answer with the correct number of significant figures.
For multistep calculations, make sure to keep track of the number of significant figures at the end of each step so we know how many significant figures to round to at the end of the entire calculation. To maintain accuracy, do not round intermediate steps.
First, do the two multiplication problems found within the parentheses.
Recall that for multiplication, the result needs to have the same number of significant figures as the factor with the least number of significant figures. Our answer from the addition should then only have 3 significant figures since that is the number of significant figures 3.14 has. To remember that the answer should only have 3 significant figures, the last significant digit will be highlighted:
Recall that for multiplication, the result needs to have the same number of significant figures as the factor with the least number of significant figures. Our answer from the addition should then only have 1 significant figure since that is the number of significant figures 10 has. To remember that the answer should only have 1 significant figure, the last significant digit will be highlighted:
Now, multiply:
Since the factor with the least number of significant figures only has 1 significant figure, the answer must also be rounded to 1 significant figure. 5570.13393 is rounded to 6000.
Example Question #31 : Calculations
Calculate and give the answer to the correct number of significant figures.
For multistep calculations, make sure to keep track of the number of significant figures at the end of each step so we know how many significant figures to round to at the end of the entire calculation. To maintain accuracy, do not round intermediate steps.
First, do the two multiplication problems found within the parentheses.
Recall that for multiplication, the result needs to have the same number of significant figures as the factor with the least number of significant figures. Our answer from the addition should then only have 1 significant figure since that is the number of significant figures 0.02 has. To remember that the answer should only have 3 significant figures, the last significant digit will be highlighted:
Next, do the addition.
Because this is addition, the result of the calculation needs to have the same number of decimal places as the number with the fewest decimal places. We need to only keep 1 decimal place because of 
Next, do the second addition.
Because this is addition, the result of the calculation needs to have the same number of decimal places as the number with the fewest decimal places. Since 10 does not have any decimal places, our final answer also cannot have any decimal places. 33.356 is rounded down to 33.
Example Question #1 : Yield And Error
3 mols of 


What is the percent conversion of 
To find the percent conversion, use the following equation:
Initially there are 3 mols of 



Plugging this into the above equation gives a conversion rate of 58%.
All AP Chemistry Resources