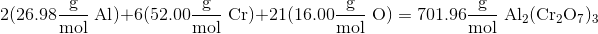All SAT II Chemistry Resources
Example Questions
Example Question #24 : Sat Subject Test In Chemistry
Suppose that 4 moles of 

Start by creating a skeleton equation for the decomposition reaction:
This can be balanced to give:
This means that 4 mol of reactant would produce 6 mol of oxygen gas. The question states that at STP, one mole of a gas takes up 22.4 L of space, so it is easy to find that 6 moles of oxygen gas would occupy a volume of 134.4 L:
Example Question #1 : Other Stoichiometric Calculations
What is the mass percent of chromium in 
Use the values in the following table for the atomic masses of the elements shown.
In order to find the mass percent of a certain atom in a particular molecule, you must know the mass the atom contributes to the molecule and the total mass of the molecule. In this case we calculate the mass percent using the equation
Calculating the mass of 





Now we need to calculate the total atomic mass of the molecule. We can do this by identifying the atomic mass of each element in the molecule, multiplying it by the number of that type of atom in the molecule, and summing the results together.
We then plug in the values into equation to find the mass percent.
Certified Tutor
Certified Tutor
All SAT II Chemistry Resources