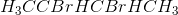All Organic Chemistry Resources
Example Questions
Example Question #3 : Help With E2 Reactions
Which of the following products will result from this reaction?







The elimination reaction (as opposed to the SN2 because of the big bulky base reagent) only proceeds with the anti-periplanar product.

Example Question #4 : Help With E2 Reactions
What is the product of the following reaction?


No reaction.

More than one of these.


Because both hydrogens neighboring the 
Example Question #3 : Help With E2 Reactions
Which of the following sets of conditions most strongly favors an E2 elimination reaction?
Weak base, polar aprotic solvent, tertiary electrophile
Strong base, polar aprotic solvent, primary electrophile
Strong base, polar aprotic solvent, tertiary electrophile
Strong base, polar protic solvent, secondary electrophile
Strong base, polar aprotic solvent, primary electrophile
Weak base, polar aprotic solvent, tertiary electrophile
For E2 reactions a tertiary electrophile > secondary electrophile > primary electrophile. A polar aprotic solvent favors E2 (remember that polar protic solvents favor E1). A strong base is needed to undergo E2.
Example Question #4 : Help With E2 Reactions
A student carried out a substitution reaction in the lab using ether as a solvent. The student began with an optically pure reactant (100% (R)-configuration) and finished with an optically pure product (100% (S)-configuration).
The reaction went through which of the following mechanisms?
Either SN1 or SN2
E1
E2
SN1
SN2
SN2
SN2 reactions result in an inversion of products, thus R-configured reactants become S-configured products and vice versa. Polar aprotic solvents such as ether favor SN2 reactions.
E1 and E2 are elimination mechanisms and the question asks for a substitution mechanism. SN1 results in racemization of products, not inversion. Thus, if the reaction had gone through SN1 we would see a mixture of R and S-configuration in the products. Additionally, polar protic solvents (not aprotic like ether) favor SN1 reactions.
Example Question #512 : Organic Chemistry
2-butene reacts with 
No reaction
Here we have a classic electrophilic addition reaction.
We must remember that a double bond is an area in a molecule that has a high electron density. As a result, double bonds can act as nucleophiles and react with good electrophiles.
In this problem, the alkene attacks the dibromide to form a bromonium ion, which is rather unstable. The bromonium ion pulls carbon's electrons toward itself, causing the carbons in the bromonium complex to have a slightly negative charge. As a result, the negatively charged bromine can attack either of the carbon atoms and, thus, we have added two bromines to our carbon chain.
Example Question #21 : Reaction Mechanisms, Energetics, And Kinematics
How many stable resonance structures are there for the following molecule (include the given structure in your total count)?

4
6
1
5
3
5
The five resonance structures of the given molecule are shown below:

Example Question #22 : Reaction Mechanisms, Energetics, And Kinematics
Rank the following compounds in order of increasing rate of electrophilic aromatic substitution.

IV, V, I, III, II
II, IV, III, I, V
II, I, III, IV, V
V, II, I, III, IV
V, I, III, IV, II
V, I, III, IV, II
The nitro substituent is strongly deactivating, ketones are moderately deactivating substituent, halides are weakly deactivating, alkyl groups are weakly activating, and amine groups are strongly activating.
Example Question #1 : Identifying Reaction Mechanisms
Which of these would react fastest with methanol via an SN1 mechanism?
All of these will react with similar rates when undergoing an SN1 reaction with methanol
An SN1 mechanism involves the leaving of the bromine in the first step and the formation of a carbocation on that carbon. The molecule with the most stable carbocation will react most quickly.
The carbocation is most stable on the most highly substituted carbon. All the of the answer options form primary or secondary cations except for one. The correct answer has a carbocation on a carbon bonded to two methyl groups and one ethyl group. The correct answer is 
Example Question #1 : Identifying Reaction Mechanisms

What is the major product of the reaction shown above?





Grignard reagents attack ketones at the site of the ketone's carbon to produce tertiary alcohols.
Example Question #2 : Identifying Reaction Mechanisms
Under which of the following sets of conditions is an SN2 reaction most favored?
Methanol solvent, tertiary electrophile, weak nucleophile
Ethanol solvent, primary electrophile, strong nucleophile
Acetone solvent, tertiary electrophile, strong nucleophile
Ether solvent, primary electrophile, strong nucleophile
DMSO solvent, primary electrophile, weak nucleophile
Ether solvent, primary electrophile, strong nucleophile
SN2 reactions require a strong nucleophile. If the the nucleophile is weak, SN1 is favored over SN2.
For SN2 reactions less substitution in the electrophile is favored (methyl > primary > secondary).
For SN2 reactions polar aprotic solvents are preferred (DMSO, acetone, DMF and ethers are common polar aprotic solvents). Polar protic solvents (ethanol, methanol) favor SN1 reactions.
Certified Tutor
Certified Tutor
All Organic Chemistry Resources