All Organic Chemistry Resources
Example Questions
Example Question #11 : Biological Molecules
The following terpene is classified as which of the following?

Diterpene
Eicosanoid
Hemiterpene
Monoterpene
Monoterpenoid
Monoterpene
The molecule's skeletal structure contains two isoprene (2-methylbutyl) units, and therefore the molecule is a monoterpene.
Example Question #11 : Help With Organic Lipids

Which of these two fatty acids has the lower melting point and why?
Stearic acid, because it contains fewer cis double bonds.
Alpha-linolenic acid, because it contains fewer cis double bonds.
They have the same melting point since both have 18 carbons.
Alpha-linolenic acid, because it contains more cis double bonds.
Stearic acid, because it contains more cis double bonds.
Alpha-linolenic acid, because it contains more cis double bonds.
Fatty acids that contain a higher degree of unsaturation (more alkene bonds) will introduce more "kinks" into the hydrocarbon chain. This "kinked" chain does not stack nicely with other fatty acids of its kind and therefore are more likely to slip past each other at lower temperatures. This is primarily due to the van der Waals forces within the unsaturated fatty acids being disrupted with the introduction of double bonds. As a result, unsaturated fatty acids generally have a lower melting point than saturated fatty acids.
Example Question #1 : Help With Organic Carbohydrates
Chemists and biochemists have many ways of representing sugars. Glucose, the most common hexose, is shown below in various linear and cyclic projections. Using the linear and cyclic projection of your choice, can you indicate which colored oxygen in the linear form corresponds to the circled hemiacetal oxygen once the cyclization reaction is complete?
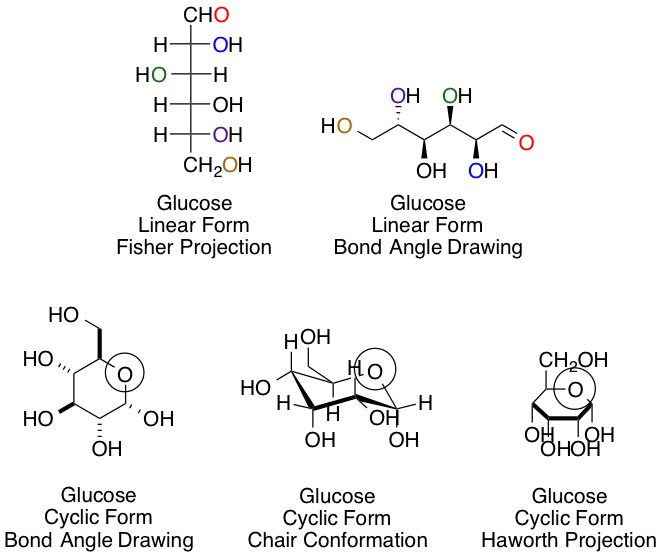
Yellow
Purple
Blue
Red
Green
Purple
This answer, regardless of your preference of projection type, is easiest to obtain using arrow pushing for the cyclization reaction to keep track of each carbon and oxygen:

The purple carbon in the linear projection ends in the circled hemiacetal position.
Example Question #2 : Identifying Monosaccharides
Which of the following structures represents the anomeric alpha ring structure of D-glucose? 






When converting a linear sugar to its ring form, a bond is formed between the oxygen attached to carbon 5 and the carbon at position 1. All hydroxyl groups that are not attached to the carbon in position 1 and are oriented to the right end up trans to the 

If the hydroxyl group attached to carbon 1 ends up trans to the 

The alpha ring structure of D-glucose bonds the carbon 1 hydroxyl group trans to the carbon 5 

Example Question #1 : Identifying Monosaccharides
Which of the following structures represents the anomeric alpha ring structure of D-galactose?







When converting a linear sugar to its ring form, a bond is formed between the oxygen attached to carbon 5 and the carbon at position 1. All hydroxyl groups that are not attached to the carbon in position 1 and are oriented to the right end up trans to the 

If the hydroxyl group attached to carbon 1 ends up trans to the 

The alpha ring structure of D-galactose bonds the carbon 1 hydroxyl group trans to the carbon 5 

Example Question #4 : Identifying Monosaccharides
Which of the following ring structures represents the anomeric alpha ring structure of D-mannose?







When converting a linear sugar to its ring form, a bond is formed between the oxygen attached to carbon 5 and the carbon at position 1. All hydroxyl groups that are not attached to the carbon in position 1 and are oriented to the right end up trans to the 

If the hydroxyl group attached to carbon 1 ends up trans to the 

The alpha ring structure of D-mannose bonds the carbon 1 hydroxyl group trans to the carbon 5 

Example Question #2 : Help With Organic Carbohydrates
Identify the aldose pictured, including its alpha or beta designation.







The structure pictured is mannose because the hydroxyl groups at carbons 2, 3, and 4 are situated cis, cis, and trans (respectively) to the 
The mannose pictured is in alpha form because the hydroxyl group at carbon 1 is trans to the 
Example Question #3 : Help With Organic Carbohydrates
The Fischer projection pictured is a form of glucose. The carbon labeled "x" is the chiral carbon farthest away from carbon 1 and the hydroxyl group connected to carbon "x" is on the right. This fact designates that the glucose as what configuration?

Pyranose
D
Beta
L
Alpha
D
The chiral carbon farthest away from carbon 1 is designated as "D" if its hydroxyl group is on the right side in the Fischer projection. In other words, this is D-glucose because the hyroxyl group on carbon "x" is oriented to the right.
Example Question #4 : Help With Organic Carbohydrates
What is the name of the aldose pictured in this Fischer projection?

L-lyxose
L-fructose
D-arabinose
L-xylose
D-ribose
D-ribose
The structure is D-ribose because it is a five-carbon aldose with the hydroxyl groups on carbons 2, 3, and 4 all on the right in the Fischer projection.
Example Question #191 : Organic Concepts
Which of the following statements is true regarding carbohydrates?
The anomeric carbon is the site of attachment from one monosaccharide to another
In nature, carbohydrates are usually found with a "D" conformation
All of these
Aldoses are more common in nature than ketoses
All of these
All of these statements are true. A carbohydrate is said to have a "D" conformation in its acyclic form when the alcohol group on the carbohydrate's top stereocenter is on the right side in a Fischer projection. Most carbohydrates that we deal with in organic chemistry are aldoses, which means that they contain an aldehyde. The anomeric carbon is the site of attachment from one monosaccharide to another, and can be used to create polysaccharides.
Certified Tutor
All Organic Chemistry Resources




