All Organic Chemistry Resources
Example Questions
Example Question #1 : Help With Ether And Ester Reactions

Predict the major product of the following reaction (the reaction is allowed to run to completion and the final step is an acid workup).
IV
I
III
II
None of these
III
Alkyl lithium (organolithium) reagents are powerful reducing agents and react much in the same manner as Grignard reagents through the formation of a carbanion. In the first step of the reaction, the carbanion attacks at the carbonyl, creating a charged intermediate, which is neutralized by reformation of the carbonyl and the release of ethoxide (the ester chain is the leaving group). However, the alkyl lithium reagent continues the reaction by attacking at the carbonyl once again. This time, there is no suitable leaving group (every group bound is a carbon chain) and the intermediate remains until a proton transfer is facilitated to neutralize the oxygen's charge.
Example Question #1 : Help With Ether And Ester Reactions
When butanol is reacted with pentanoic acid in the presence of an acid catalyst, what would be the expected major product?
Butyl pentanoate
Butanoic acid
Pentene
Butene
Butyl pentanoate
To answer this question, it's important to consider what types of molecules are reacting. The pentanoic acid is a carboxylic acid, and the butanol is an alcohol. When carboxylic acids are reacted with alcohols in the presence of an acid catalyst, the result is an ester.
Example Question #571 : Organic Chemistry

What is the product of the given reaction?
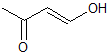





This is a basic Claisen condensation reaction in which the 
 .
.
The attack at the ester carbonyl leads to the leaving of 
Example Question #2 : Help With Ether And Ester Reactions
What is the product of the following reaction?


II and III
II only
I and II
IV only
II and III
Treatment of ethyl acetate with methoxide will yield the enolate ion (II). However, side trans-esterification reactions will also result in formation of product III.
Certified Tutor
All Organic Chemistry Resources




