All MCAT Physical Resources
Example Questions
Example Question #94 : Mcat Physical Sciences
Halogens start out as gases (fluorine and chlorine), but transition to liquid (bromine) and then to solid (iodine) when moving down group 17 of the periodic table. This is because __________.
the larger molecules have larger electronegativities, making them cluster closer together with one another
London dispersion forces increase as the molecules become larger
the atoms lose their valence electrons more easily
the atoms become heavier
London dispersion forces increase as the molecules become larger
The effects of London dispersion forces are best demonstrated by viewing the halogen group. The lighter halogens are gaseous at room temperature, but transition to liquid and then to solid when moving down the group (bromine is a liquid, and iodine is a solid). When moving down the group, the halogens become much larger. Larger atoms experience greater London dispersion forces due to increased surface contact and greater polarizability. These increased London dispersion forces are enough to raise the boiling points of the larger atoms, causing them to exist as liquids of gases at room temperature.
While it is true that the larger atoms are heavier and will lose their valence electrons more readily, these factors do not impact the phase of the compound.
Example Question #852 : Mcat Physical Sciences

Epimers
Isotopes
Isomers
Resonance structures
Resonance structures
The answer is resonance structures. When different Lewis structures can be drawn for a single molecule, the molecule exists as a composite of these structures, which are called resonance structures.
Isotopes refers to multiple nuclear compositions for a single element, based on varying numbers of neutrons. Isomers are different configurations of a given molecular formula, based on geometry and orientation. Epimers are a specific class of isomers involving a single stereocenter.
Example Question #1 : Lewis Dot Structures
Diffusion can be defined as the net transfer of molecules down a gradient of differing concentrations. This is a passive and spontaneous process and relies on the random movement of molecules and Brownian motion. Diffusion is an important biological process, especially in the respiratory system where oxygen diffuses from alveoli, the basic unit of lung mechanics, to red blood cells in the capillaries.
Figure 1 depicts this process, showing an alveoli separated from neighboring cells by a capillary with red blood cells. The partial pressures of oxygen and carbon dioxide are given. One such equation used in determining gas exchange is Fick's law, given by:
ΔV = (Area/Thickness) · Dgas · (P1 – P2)
Where ΔV is flow rate and area and thickness refer to the permeable membrane through which the gas passes, in this case, the wall of the avlveoli. P1 and P2 refer to the partial pressures upstream and downstream, respectively. Further, Dgas, the diffusion constant of the gas, is defined as:
Dgas = Solubility / (Molecular Weight)^(1/2)
How many total valence electrons does carbon dioxide contain?
22
10
24
16
16
This is a straightforward question that has little to do with the passage, but it's a concept almost certainly to be seen on the MCAT.
Make sure to understand the concept of valence electrons and what that number may tell you about bonding properties. Questions like these are essentially "freebies," and should be answered without hesitation.
Example Question #853 : Mcat Physical Sciences
How many valence electrons do boron and nitrogen have?
Boron has three valence electrons.
Nitrogen has four valence electrons.
Boron has three valence electrons.
Nitrogen has five valence electrons.
Boron has seven valence electrons.
Nitrogen has eight valence electrons.
Boron has five valence electrons.
Nitrogen has three valence electrons.
Boron has three valence electrons.
Nitrogen has five valence electrons.
To determine the number of electrons an atom has, you must look at which column the atom is in on the periodic table. Boron is in column 3A, so it has three valence electrons. Nitrogen is in column 5A, so it has five valence electrons.
You should be familiar with common elements, like nitrogen, without looking at the periodic table. This will save you time on the exam.
Example Question #21 : Compounds, Molecules, And Bonds
Which statement best describes VSEPR theory?
The repulsion between electrons helps determine the geometry of covalent molecules
Covalent bonds are formed by overlapping valence electron shells
The repulsion between atoms helps determine the polarity of molecules
The repulsion between protons in an atom's nucleus determines its size
Molecular shapes are determined according to which orbitals are energetically accessible for bonding
The repulsion between electrons helps determine the geometry of covalent molecules
The idea of VSEPR (valence shell electron pair repulsion) theory is that valence electron pairs repel each other, arranging themselves into positions that minimize their repulsions by maximizing the distance between them. The positions of these electron pairs then determine the overall geometry of the molecule. Molecular geometry is thus determined by the arrangement of electrons and nuclei such that the electrons are as far from one another as possible, while remaining as close to the positively charged nucleus as possible.
Example Question #2 : Vsepr Geometry
Which of the following compounds has a molecular tetrahedral geometry?
Of the available answer choices, only 



Example Question #22 : Compounds, Molecules, And Bonds
What is the molecular geometry of sulfur hexafluoride?
Square planar
Tetrahedral
Octahedral
Trigonal bipyramidal
Octahedral
Sulfur hexaflouride (SF6) is an example of octahedral geometry, as it follows the skeleton of AX6E0 format. A refers to sulfur, X to fluorine, and E to the lone pair electrons.
Square planar has an AX4E2 format, while tetrahedral and trigonal bipyramidal follow AX4 and AX5 formats, respectively.
Example Question #2 : Diagrams And Geometry
Which of the following is not the correct geometric configuration for the given molecule?
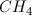





Recall the following relationships between geometry and number of pairs of electrons on the central atom.
2: linear
3: trigonal planar
4: tetrahedral
5: trigonal bipyriamidal
6: octahedral
To visualize the geometry, we need to think of how many electron pairs are on the central atom. Drawing Lewis dot diagrams may be helpful here. None of the answer choices has lone central electron pairs, with the exception of water, so the number of atoms bound to the central atom is the same as the number of central electron pairs.
The only one that does not match up with the correct geometry is SF6, which is actually octahedral since it has six central electron pairs. In a water molecule, the central oxygen has six valence electrons, plus one from each bond with hydrogen, for a total of eight central electrons and four central electron pairs. So, this geometry is a variation on the tetrahedral form (bent), in which two central electron pairs are not bound.
Example Question #23 : Compounds, Molecules, And Bonds
Which of the following molecules will have the smallest bond angles?
In order to determine which molecule will have the smallest bond angle(s), make sure to factor in both the number of atoms around the central atom as well any lone pairs on the central atom. 


Example Question #4 : Vsepr Geometry
The geometry of a certain molecule with the general formula 
Octahedral geometry always corresponds to the 








Certified Tutor
All MCAT Physical Resources















