All MCAT Biology Resources
Example Questions
Example Question #62 : Organic Chemistry, Biochemistry, And Metabolism
What reagent would be best suited to accomplish the transformation shown below?

Possible Answers:
Methyl magnesium bromide,
Correct answer:
Explanation:
Reduction of an ester to an alcohol requires a strong reducing agent, such as 

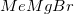
Rahul Patel
Certified Tutor
Certified Tutor
Rutgers University-Newark, Bachelors, Biology, General. University of Medicine and Dentistry of New Jersey, Current Grad Stud...
All MCAT Biology Resources
Popular Subjects
Math Tutors in Philadelphia, Reading Tutors in Chicago, Algebra Tutors in Miami, Physics Tutors in Boston, Spanish Tutors in Phoenix, English Tutors in San Diego, French Tutors in Los Angeles, Spanish Tutors in San Francisco-Bay Area, Biology Tutors in Washington DC, English Tutors in Los Angeles
Popular Courses & Classes
LSAT Courses & Classes in Boston, SAT Courses & Classes in Washington DC, SSAT Courses & Classes in Denver, LSAT Courses & Classes in Los Angeles, GMAT Courses & Classes in Boston, MCAT Courses & Classes in Atlanta, GRE Courses & Classes in Atlanta, MCAT Courses & Classes in Dallas Fort Worth, GRE Courses & Classes in Miami, Spanish Courses & Classes in New York City
Popular Test Prep
GMAT Test Prep in New York City, LSAT Test Prep in San Francisco-Bay Area, SAT Test Prep in Philadelphia, MCAT Test Prep in Dallas Fort Worth, SAT Test Prep in Atlanta, SSAT Test Prep in Boston, SSAT Test Prep in Phoenix, SSAT Test Prep in Miami, SAT Test Prep in Houston, LSAT Test Prep in Los Angeles









