All MCAT Biology Resources
Example Questions
Example Question #11 : Lipids
Drain cleaners are a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
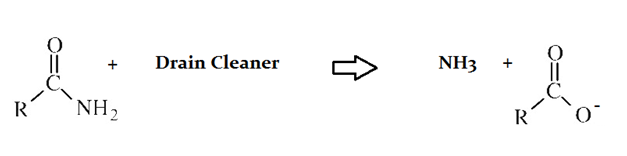
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
The drain cleaner in Reaction 1 was used to break down fats as well as the protein depicted. How would the fats be most different in molecular structure?
They would be more reactive than the proteins.
They would be more oxidized than the proteins.
They would totally lack any polar bonds.
They would be more acidic than the proteins.
They would have much lower overall polarity in their bonds.
They would have much lower overall polarity in their bonds.
Fats are less reactive, more reduced, and less acidic than proteins. They have much lower overall polarity, but do contain polar bonds, such as between C and O.
Example Question #281 : Organic Chemistry, Biochemistry, And Metabolism
Water often acts as a reactant or solvent in biological reactions. Which of the following cellular components would not be sufficiently solvated in the body?
Nucleotides
Carbohydrates
Lipids
Amino acids
Lipids
A molecule is solvated when it is surrounded by water molecules, and separated from the other molecules in the body. This separation is possible because of charge or polarity present in the molecule, which causes it to be attractive to water.
Lipids have very low solubility in water due to their nonpolarity. As a result, we conclude that lipids would not be properly solvated in the body, and would instead be clustered together by water molecules.
Certified Tutor
All MCAT Biology Resources