All MCAT Biology Resources
Example Questions
Example Question #1 : Covalent Bonds And Hybrid Orbitals
Under normal circumstances, which of the following carbons will be sp3 hybridized?
Carbocation
Free radical carbon
Carbon involved in a double bond
Carbanion
Carbanion
Carbanions are usually sp3 hybridized. Free radical carbons, carbocations, and double bonded carbons are all sp2 hybridized.
Example Question #1521 : Mcat Biological Sciences
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
The bond that is present between the carbon atom and the carbonyl oxygen atom in carbonc acid is best described as having which of the following?
Two sigma bonds
One pi bond, one sigma bond
Resonance only when the acid is protonated
Two bonds with half pi character and half sigma chacter
Two pi bonds
One pi bond, one sigma bond
The bond in question is a double bond, and thus is composed of one sigma and one pi bond.
Example Question #1521 : Mcat Biological Sciences
How many pi bonds are present in benzylamine?
Three
Four
Eight
Seventeen
Three
Benzylamine consists of a benzene ring with a -CH2NH2 substituent. The benzene ring contains three double bonds, and the substituent has none.
Each bond in a compound represents a sigma bond. Each additional bond represents a pi bond; thus, double binds result from one sigma and one pi bond, and triple bonds from one sigma and two pi bonds. In benzylamine, there are three double bonds and seventeen total bonds, three of which are double bonds. The compound will have a total of seventeen sigma bonds and three pi bonds.
Example Question #2 : Covalent Bonds And Hybrid Orbitals
Compound A has a molecular formula of 

One
Two
Zero
Four
Five
Two
Recall the formula for degrees of unsaturation as the sum of the number of pi bonds and the number of rings. This value is equivalent to the below formula.
C is the number of carbons, H is the number of hydrogens, X is the number of halogens, and N is the number of nitrogens. Using this formula, compound A has four degrees of unsaturation, while the product only has two.
The reaction described is a hydrogenation reaction, which will reduce double bonds. The reaction saturates two pi bonds of compound A, meaning the remaining degrees of unsaturation in that compound have to come from two rings. Essentially, due to the reaction given, we know that the product contains no double bonds but still has two degrees of unsaturation. These unsaturation points must correspond to rings.
Example Question #1 : Covalent Bonds And Hybrid Orbitals
How many bonds are present in this molecule?
Five sigma bonds and two pi bonds
Four sigma bonds and one pi bond
Three sigma bonds and three pi bonds
Five sigma bonds and one pi bond
Four sigma bonds and two pi bonds
Five sigma bonds and one pi bond
The molecule has four single bonds and one double bond.
The first carbon forms two single bonds with the two hydrogen atoms, and a double bond to the second carbon. The second carbon has single bonds with the third hydrogen and the chlorine atom. In total, each carbon has two single bonds and one double bond (to each other), for a total of four single bonds and one double bond.
Each single bond contains one sigma bond and zero pi bonds. Each double bond contains one sigma bond and one pi bond. This molecule has five sigma bonds, and one pi bond.
Example Question #1521 : Mcat Biological Sciences
Which of the following compounds has five degrees of unsaturation?

Compounds B and C
Compounds A, B, and C
Compound A
Compounds A and C
Compound C
Compounds A and C
A degree of unsaturation results from either a ring or a double bond. In the case of a benzene ring or phenyl substituent, four degrees of unsaturation are present: the ring itself and the three included double bonds.
Compounds A, B, and C all possess a benzene ring, but compounds A and C also have one additional degree of unsaturation. Compound A contains a carbon-oxygen double bond in the ketone group and compound C contains a carbon-carbon double bond in the alkene group. In addition to the four degrees from the ring, these additions bring their total degrees of unsaturation to five. Compound B has no additional double bond, and therefore has four degrees of unsaturation.
Example Question #1522 : Mcat Biological Sciences
The degree of unsaturation for ephedrine (shown below) is __________.

The degree of unsaturation is equal to the number of rings plus the number of pi bonds in a molecule. Ephedrine has one ring and three pi bonds, so its degree of unsaturation is four.
To arrive at this answer, one could also use the formula below, where 



For ephedrine, 



Example Question #1 : Organic Functional Groups And Molecules
Which compound(s) shown above is(are) aromatic?
D
A, B, and C
A and C
A
A
For a compound to be considered aromatic, it must be flat, cyclic, and conjugated and it must obey Huckel's rule. Huckel's rule states that an aromatic compound must have 
Example Question #1523 : Mcat Biological Sciences
How many 
Seven
Twelve
Three
Six
Two
Seven
Acetic acid has the formula 
Whenever two atoms are directly bonded to one another, a 



Example Question #1523 : Mcat Biological Sciences
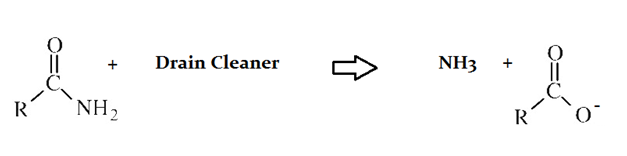
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
In the carbonyl bonds of the preceeding passage __________.
The carbon exhibits sp3 hybridization
The carbon exhibits sp2 hybridization
Sigma bonds exist above and below the plane of the pi bonds
Pi bonds result from pure s orbital overlap
The carbon exhibits sp hybridization
The carbon exhibits sp2 hybridization
The carbon at the center of a carbonyl group bonds with three sigma bonds, and one pi bond. The pi bond exists above and below the plane of the sigma bond. This carbon thus shows sp2 hybridization.
Certified Tutor
All MCAT Biology Resources