All MCAT Biology Resources
Example Questions
Example Question #1531 : Mcat Biological Sciences
Which organelle would have the most negative effect if its membrane were damaged?
Lysosomes
Golgi body
Mitochondria
Ribosomes
Chloroplasts
Lysosomes
The lysosomes contain an acidic environment and digestive enzymes. Damage to the membrane would allow hydrogen ions and these enzymes to escape into the cytoplasm of the cell, where they would do damage to the other cellular components.
Damage to a mitochondrion or chloroplast would affect energy production in the cell, but would not actively cause damage. Ribosomes don't have membranes.
Example Question #141 : Organic Chemistry, Biochemistry, And Metabolism
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
What is the predicted molecular orbital hybridization state of the carbon in carbonic acid?
sp2
Pi
Sigma
sp
sp3
sp2
Carbons bound via one double bond are sp2 hybridized, as long as the remaining two bonds are each sigma bonds. Take the number of sigma bonds and subtract one for your exponent in the spx expression.
Example Question #141 : Organic Chemistry, Biochemistry, And Metabolism
When formic acid is completely reduced, methanol is formed.
What is the hybridization of the carbon in formic acid, compared to the carbon in methanol?
The carboxylic acid has sp2 hybridization and the alcohol has sp2 hybridization.
The carboxylic acid has sp3 hybridization and the alcohol has sp2 hybridization.
The carboxylic acid has sp3 hybridization and the alcohol has sp3 hybridization.
The carboxylic acid has sp2 hybridization and the alcohol has sp3 hybridization.
The carboxylic acid has sp2 hybridization and the alcohol has sp3 hybridization.
In order to find the hybridization of an atom, simply count the number of sigma bonds and lone pair electrons around the atom. The carbon in formic acid is double bonded to an oxygen, and has two single bonds. This means that it has sp2 hybridization. Upon being reduced to methanol, the carbon now has four single bonds surrounding it. As a result, the carbon now has sp3 hybridization.
Remember that a triple bond corresponds to sp hydrization, a double bond to sp2, and single bonds to sp3 for a carbon atom.
Example Question #1534 : Mcat Biological Sciences
One component of the immune system is the neutrophil, a professional phagocyte that consumes invading cells. The neutrophil is ferried to the site of infection via the blood as pre-neutrophils, or monocytes, ready to differentiate as needed to defend their host.
In order to leave the blood and migrate to the tissues, where infection is active, the monocyte undergoes a process called diapedesis. Diapedesis is a process of extravasation, where the monocyte leaves the circulation by moving in between endothelial cells, enters the tissue, and matures into a neutrophil.
Diapedesis is mediated by a class of proteins called selectins, present on the monocyte membrane and the endothelium. These selectins interact, attract the monocyte to the endothelium, and allow the monocytes to roll along the endothelium until they are able to complete diapedesis by leaving the vasculature and entering the tissues.
The image below shows monocytes moving in the blood vessel, "rolling" along the vessel wall, and eventually leaving the vessel to migrate to the site of infection.

Neutrophils make use of radical species to digest phagocytosed material. Which of the following is true of radical reactions?
Tertiary radical carbons are the least stable
Radical carbons are tetrahedral
Radical carbons have excess negative charge
Radical carbons are sp2 hybridized
Radical reactions always terminate by binding to the wall of the reaction container
Radical carbons are sp2 hybridized
Radical reactions have an sp2, trigonal planer carbon radical intermediate. Carbon radicals are most stable as tertiary species.
Example Question #1 : Molecules And Compounds
2-butyne contains all of the following types of bonds except __________.
2-butyne has the following chemical structure.
The end carbons have 


Example Question #143 : Organic Chemistry, Biochemistry, And Metabolism
What carbon hybridizations can be found in one molecule of acetic acid?





Acetic acid has the formula 
Acetic acid has two carbons in the molecule. The carbonyl carbon is the carbon that is double bonded to oxygen. This double bond gives the carbonyl carbon

Example Question #143 : Organic Chemistry, Biochemistry, And Metabolism
Which of the following statements about the character of a bond is true?
The more "s" character a bond has, the longer the bond becomes
The more "s" character a bond has, the more stable it is
The more "s" character a bond has, the higher the energy in the bond
The more "s" character a bond has, the weaker it becomes
The more "s" character a bond has, the more stable it is
The character of a hybrid orbital is defined as the extent to which it resembles the unhybridized orbitals that created it. For example, an 


Example Question #141 : Organic Chemistry, Biochemistry, And Metabolism
Which of the letters above point at an atom that is 
B, C, and D
B and C
A and C
C
A and C
The answer is arrows A and C. The carbon that is pointed to by arrow C is 



We can quickly tell the hybridization of atoms by observing their double bonds and unbonded electrons. As a rule of thumb, any carbon, nitrogen, or oxygen involved in a double bond will be 


Example Question #1536 : Mcat Biological Sciences
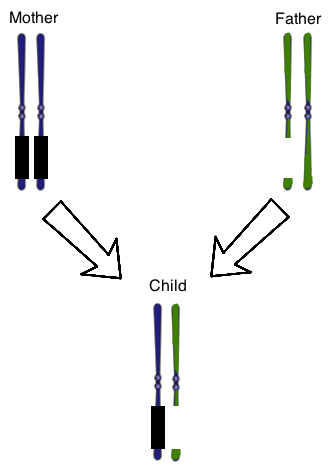
The concept of genomic imprinting is important in human genetics. In genomic imprinting, a certain region of DNA is only expressed by one of the two chromosomes that make up a typical homologous pair. In healthy individuals, genomic imprinting results in the silencing of genes in a certain section of the maternal chromosome 15. The DNA in this part of the chromosome is "turned off" by the addition of methyl groups to the DNA molecule. Healthy people will thus only have expression of this section of chromosome 15 from paternally-derived DNA.
The two classic human diseases that illustrate defects in genomic imprinting are Prader-Willi and Angelman Syndromes. In Prader-Willi Syndrome, the section of paternal chromosome 15 that is usually expressed is disrupted, such as by a chromosomal deletion. In Angelman Syndrome, maternal genes in this section are deleted, while paternal genes are silenced. Prader-Willi Syndrome is thus closely linked to paternal inheritance, while Angelman Syndrome is linked to maternal inheritance.
Figure 1 shows the chromosome 15 homologous pair for a child with Prader-Willi Syndrome. The parental chromosomes are also shown. The genes on the mother’s chromosomes are silenced normally, as represented by the black boxes. At once, there is also a chromosomal deletion on one of the paternal chromosomes. The result is that the child does not have any genes expressed that are normally found on that region of this chromosome.
The passage indicates that genomic imprinting relies on silencing genes by adding methyl groups to DNA sequences. Which of the following is true of methyl groups?
The central carbon is 
The methyl group forms ionic bonds with DNA
The methyl group forms hydrogen bonds with DNA
The central carbon is 
The central carbon is 
The central carbon is 
Methyl groups are 


Note that methylation is a covalent interaction, making it much more permanent than dipole interactions between DNA and histones. This allows methylation to have long-term effects, and is one of the principles of epigenetic regulation and inheritance.
Example Question #3 : Covalent Bonding
For the compound shown below, the hybridization for carbon A is __________ and the hybridization for carbon B is __________.

Carbon A is 

Keep in mind that a carbon involved in a triple bond will always be 


Certified Tutor
All MCAT Biology Resources


















