All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #2 : Mcat Biological Sciences
A student has just finished running a reaction for organic chemistry lab. The reaction has produced a primary amine, as well as multiple dialkyl ether byproducts. The student has 1N aqueous HCl, 1N aqueous NaOH, water, and ethyl acetate at his disposal for purification. After diluting the crude reaction mixture with ethyl acetate, which of the following extraction methods will successfully isolate the amine product?
1) Extract the organic phase with 1N aqueous NaOH.
2) Discard the organic phase.
3) Neutralize the aqueous extractions with 1N aqueous HCl.
4) Extract the neutralized aqueous phase with ethyl acetate.
1) Extract the crude mixture with 1N aqueous NaOH.
2) Discard the organic phase.
3) Neutralize the aqueous extracts with 1N aqueous HCl.
4) Extract the neutralized aqueous phase with ethyl acetate.
1) Extract the crude mixture with 1N aqueous HCl.
2) Discard the organic phase.
3) Extract the aqueous phase with ethyl acetate.
1) Wash the crude mixture with water.
2) Discard the organic phase.
3) Neutralize the aqueous washings with ethyl acetate.
1) Extract the crude mixture with 1N aqueous HCl.
2) Discard the organic phase.
3) Neutralize the aqueous extracts with 1N aqueous NaOH.
4) Extract the neutralized aqueous phase with ethyl acetate.
1) Extract the crude mixture with 1N aqueous HCl.
2) Discard the organic phase.
3) Neutralize the aqueous extracts with 1N aqueous NaOH.
4) Extract the neutralized aqueous phase with ethyl acetate.
To isolate the amine, it is first necessary to treat it with acid to form an ammonium salt. This would go into the aqueous phase along with the HCl, while the dialkyl ether byproducts would remain behind in the organic phase of the crude mixture.
After discarding the organic phase, treating the aqueous phase with 1N NaOH would convert the ammonium salt back to the free amine, which could then be extracted with ethyl acetate.
As for the other answers, extracting the organic phase with aqueous NaOH would leave the amine behind in the organic phase, as would washing with water. Also, the protonated amine would have to be neutralized in the aqueous phase with NaOH in order for it to be extracted back into ethyl acetate.
Example Question #1 : Organic Chemistry, Biochemistry, And Metabolism
A first-year graduate student treats compound A (below) with aqueous sodium hydroxide and heats the reaction for one hour. After cooling down the reaction, what must he do to isolate the desired carboxylic acid product?

Treat the aqueous phase with dilute acid, and then extract repeatedly with ethyl acetate
Add more sodium hydroxide solution, then add brine, and finally extract with ethyl acetate
Concentrate the crude reaction mixture
Extract the reaction mixture with ethyl acetate
Add dilute acid to neutralize any base, and then concentrate the reaction mixture
Treat the aqueous phase with dilute acid, and then extract repeatedly with ethyl acetate
During the reaction, the ester is converted to a carboxylic acid, however, because of the basic conditions the acid would be deprotonated to form a carboxylate salt. This salt would be soluble in the aqueous phase, and would have to be protonated by adding dilute acid to convert it into a neutral molecule. We want to isolate the carboxylic acid product via extraction, forcing it from the aqueous phase to the organic phase. Only when the product is neutral can it be extracted by organic ethyl acetate.
Simply concentrating the reaction mixture, either by itself or after neutralizing any base with acid, would be insufficient because residual inorganic salts would remain with the desired carboxylic acid. Likewise, extracting the reaction with ethyl acetate without adding any acid would not extract the product, as it would remain in the aqueous phase as a carboxylate salt.
Example Question #4 : Mcat Biological Sciences
When would it be appropriate to use extraction in order to separate compounds in a solution?
When the compounds have differing conjugated double bond lengths, but similar molecular weights.
When the compounds have similar polarities, but differing solubilities.
When the compounds have similar molecular weights, but differing polarities.
When the compounds have differing molecular weights, but similar solubilities.
When the compounds have similar polarities, but differing solubilities.
Extraction is a useful separation technique when there is a mixture of compounds in a solution that have similar polarities, but different solubilities. The three-step process of extraction can take advantage of different solubilities by introducing the mixture to different acidic and basic conditions.
Example Question #5 : Mcat Biological Sciences
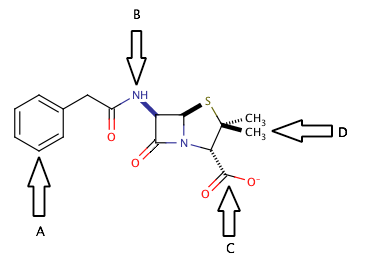
Shown above is the chemical structure for penicillin. Which of the arrows points to a functional group that could be used to extract penicillin into the aqueous layer during liquid-liquid extraction?
C only
A and B
B and C
B only
C only
The correct answer is arrow C. Separation into the aqueous layer during liquid-liquid extraction is most easily accomplished by creating a salt somewhere in the molecule. There are two common targets for creating salts in organic molecules. The first target is to create a carboxylate salt, by the addition of an inorganic base (such as NaOH) to a carboxylic acid or an existing carboxylate group, as shown in the diagram at arrow C.
Another common target for forming a salt would be to protonate the nitrogens of the molecule. Addition of an inorganic acid (such as HCl) would be a good way to protonate the nitrogens and also provide a negatively charged ion that would form the salt. In this case, however, the nitrogen at arrow B is involved in an amide functional group, and cannot be protonated. For the nitrogen to be useful in extraction, it must be part of an amine group.
The main point to take away from here is that during liquid-liquid extraction, the goal is to separate compounds into aqueous and organic layers and isolate the desired product alone in one of these layers. Ions and salts will generally be extracted into the aqueous layer, where nonpolar large structures will generally be extracted into the organic layer. Look for areas on the molecule that are susceptible to acid/base reactions, and you will find a good target for creating an ion for liquid-liquid extraction.
Example Question #91 : Analytical Chemistry
Extraction is the process of removing compounds from a solution by taking advantage of varying solubilities. The process of extraction for a mixture of diethylamine, phenol, acetic acid, and ammonia involves three steps.
1. Add a strong acid and shake the solution. Let the aqueous layer drain off.
2. Add a weak base and shake the solution. Let the aqueous layer drain off.
3. Add a strong base and shake the solution. Let the aqueous layer drain off.
Which of the following compounds would be the last to enter the aqueous phase using this technique?
Water
Ammonia
Phenol
Acetic acid
Diethylamine
Phenol
Think about what will be reacting with each substance as it is introduced to the solution.
First, a strong acid is added. This will make all bases in the solution (ammonia and diethylamine) become protonated, allowing them to become soluble. They will transition to the aqueous phase and be removed.
Next, a weak base is added. Only carboxylic acids will be deprotonated by this addition, gaining a charge and transitioning to the aqueous phase. Acetic acid will be extracted.
Finally, a strong base will deprotonate the weak acids, such as phenol. As a result, phenol will be the last molecule to leave the solution.
Example Question #11 : Mcat Biological Sciences
Which of the following mixtures would not be adequately separated via distillation?
Racemic mixture
A solution of alcohols of different sizes
A solution of polar and nonpolar compounds
A solution containing three diastereomers
Racemic mixture
Distillation involves the separation of compounds by taking advantage of their different boiling points. Mixtures with similar boiling points would not be adequately separated using distillation.
A racemic mixture is a mixture of enantiomers, which have identical boiling points. As a result, distillation would not be effective for separating a racemic mixture.
Diastereomers will have differing physical properties and can be separated by boiling point. Larger alkane chains result in higher boiling points, allowing separation of different sized alcohols by distillation. Polar compounds will have greater intermolecular forces, generally leading to higher boiling points, and allowing them to be separated from nonpolar compounds.
Certified Tutor
All GRE Subject Test: Chemistry Resources