All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #61 : General Chemistry
What hybrid orbitals are found in a molecule of water?
sp
sp2
sp3d
sp3
sp3
The hybrid orbitals for a molecule are determined by the number of atoms and lone pairs attached to the central atom. These two numbers are added to each other in order to determine the hybridization of the bonds. If there are two atoms or lone pairs attached to the central atom, it will exhibit sp hybridization. If there are three total atoms or lone pairs attached to the central atom, it will display sp2 hybridization. Water has two hydrogens and two lone pairs attached. This means that water has sp3 hybridization.
Example Question #2 : Nuclear Chemistry

What is the hybridization of the indicated carbon in the given molecule?
sp2d
sp
sp3
sp2
spf
sp3
The correct answer is sp3. First knock out any answers that aren't actual orbitals (for example spf skipped the d orbital entirely). After doing this, tackle the problem using the following guidelines:
Electrons are contained within orbitals that carry different names (s, p, d, f) based on how far they are from the nucleus. The further these atomic orbitals are away from the nucleus, the higher in energy they are. When two atoms come together to form a covalent bond, their outer most orbitals overlap and the electrons within them are shared. Two s atomic orbitals overlapping would be lower energy (and thus more stable) than say if an s and a p orbital or two p orbitals overlapped.
The geometry associated with compounds and the strengths of each bond would then be varied based on which atomic orbitals decided to overlap, but in simple molecules like methane 
When determining how many atomic orbitals are being used in covalent bonds for an atom within a molecule, you first count how many sigma (single) bonds there are and ignore any pi (double) bonds. If there are 3 sigma bonds, that means you have s + p + p atomic orbitals combining to form 3 sp3 hybrid orbitals (and so the hybridization of that atom would be sp3). We ignore pi (double) bonds because they are formed by the overlap to 2 p atomic orbitals. Also don’t forget to count invisible hydrogens that are not always drawn in.
Example Question #62 : General Chemistry

What is the hybridization of the carbon being pointed to by the arrow?
sp3df
sp
sp3d
sp2
sp3
sp3
The correct answer is sp3. First knock out any answers that make no sense for carbon based upon its valency (aka carbon doesn't have access to higher energy orbitals like d or f). After doing this you can tackle the problem using the following rules.
Hybridization theory confuses a lot of people. Here is the main concept quickly summed up. Electrons are contained within orbitals which carry different names (s, p, d, f) based upon how far they are from the nucleus. The further these atomic orbitals are away from the nucleus, the higher in energy they are. When two atoms come together to form a covalent bond, their outer most orbitals overlap and the electrons within them are shared. Two s atomic orbitals overlapping would be lower energy (and thus more stable) than say if an s and a p orbital or two p orbitals overlapped.
The geometry associated with compounds and the strengths of each bond would then be varied based on which atomic orbitals decided to overlap, but in simple molecules like methane (CH4) the bond strengths are all equivalent and the molecular geometry is quite uniform/precise. It was theorized that all the orbitals involved in bonding might merge and form new shapes called hybrid orbitals. This allowed all the hybrid orbitals to obtain the same energy level as seen in nature. So if an s atomic orbital and a p atomic orbital combined, they would form an sp hybrid orbital with combined properties of both those atomic orbitals.
When determining how many atomic orbitals are being used in covalent bonds for an atom within a molecule, you first count how many sigma (single) bonds there are and ignore any pi (double) bonds. If there are 3 sigma bonds, that means you have s + p + p atomic orbitals combining to form 3 sp3 hybrid orbitals (and so the hybridization of that atom would be sp3). We ignore pi (double) bonds because they are formed by the overlap to 2 p atomic orbitals. Also don’t forget to count invisible hydrogens that are not always drawn in!!! With these few rules you should never get a hybridization question wrong.
Example Question #61 : Gre Subject Test: Chemistry

What is the hybridization of the indicated carbon in the given molecule?
sp2
sp
sp3d
sppp
sp3
sp2
The correct answer is sp2. First knock out any answers that aren't actual orbitals (for example sppp is not the proper notation so you could knock that out as an answer choice). After doing this you can tackle the problem using the following summary:
Electrons are contained within orbitals that carry different names (s, p, d, f) based on how far they are from the nucleus. The further these atomic orbitals are away from the nucleus, the higher in energy they are. When two atoms come together to form a covalent bond, their outer most orbitals overlap and the electrons within them are shared. Two s atomic orbitals overlapping would be lower energy (and thus more stable) than say if an s and a p orbital or two p orbitals overlapped.
The geometry associated with compounds and the strengths of each bond would then be varied based on which atomic orbitals decided to overlap, but in simple molecules like methane 
When determining how many atomic orbitals are being used in covalent bonds for an atom within a molecule, you first count how many sigma (single) bonds there are and ignore any pi (double) bonds. If there are 3 sigma bonds, that means you have s + p + p atomic orbitals combining to form 3 sp3 hybrid orbitals (and so the hybridization of that atom would be sp3). We ignore pi (double) bonds because they are formed by the overlap to 2 p atomic orbitals. Also don’t forget to count invisible hydrogens that are not always drawn in.
Example Question #61 : Gre Subject Test: Chemistry

What is the hybridization of the indicated carbon in the given molecule?
sp2
sp3d
sp
sp3
sdp
sp2
The correct answer is sp2. First knock out any answers that aren't actual orbitals (for example sdp has the orbitals out of order of their respective energy levels). After doing this, tackle the problem using the following guidelines:
Electrons are contained within orbitals that carry different names (s, p, d, f) based on how far they are from the nucleus. The further these atomic orbitals are away from the nucleus, the higher in energy they are. When two atoms come together to form a covalent bond, their outer most orbitals overlap and the electrons within them are shared. Two s atomic orbitals overlapping would be lower energy (and thus more stable) than say if an s and a p orbital or two p orbitals overlapped.
The geometry associated with compounds and the strengths of each bond would then be varied based on which atomic orbitals decided to overlap, but in simple molecules like methane 
When determining how many atomic orbitals are being used in covalent bonds for an atom within a molecule, you first count how many sigma (single) bonds there are and ignore any pi (double) bonds. If there are 3 sigma bonds, that means you have s + p + p atomic orbitals combining to form 3 sp2 hybrid orbitals (and so the hybridization of that atom would be sp2). We ignore pi (double) bonds because they are formed by the overlap to 2 p atomic orbitals. Also don’t forget to count invisible hydrogens that are not always drawn in.
*If this is a little confusing to think of at the moment, you can always calculate:
steric number = # of atoms bonded (to the atom in question) + lone pairs on that atom
If the steric number is 4 = sp3, if 3 = sp2, if 2 = sp.
Example Question #3 : Nuclear Chemistry

Note the indicated carbon. What bond angle is this carbon experiencing?
The correct answer is 
steric number = # of atoms bonded (to the atom in question) + lone pairs on that atom. If the steric number is 4 = sp3, if 3 = sp2, if 2 = sp.
Next with your understanding of hybridization theory, you know that carbons that are sp hybridized are linear by nature (a common linear molecule example is carbon dioxide). If the geometry of that carbon is linear then that means the bond angles it is experiencing must be 
Example Question #1 : Nuclear Chemistry
How many electrons can fit in the 
Six
Eighteen
Fourteen
Eight
Ten
Eighteen
An 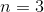
This question is best solved by using quantum numbers. We are given the principle quantum number. Using this, we can work down to determine how many electrons can fit in this shell. The rules for quantum numbers are given below.
If the principle quantum number is three, then the next quantum number can be zero, one, or two. These correspond to the s, p, and d subshells, respectively.
After that, the next quantum number describes the orbitals within the subshells. Each orbital can carry two electrons, according to the final quantum number.
In total, there are eighteen electrons allowed in all subshells of the third energy level.
Example Question #31 : Nuclear Chemistry And Electrons
What is the electron configuration of potassium after it obtains a +1 charge?
[Ar]4s23d4
[Ar]4s2
[Ne]3s1
[Ar]
[Ar]4s1
[Ar]
Potassium (K) is orignially in the electron configuration of [Ar]4s1. To obtain a +1 charge it loses an electron, resulting in a configuration of [Ar].
Example Question #1 : Electron Configuration
Which of the following species is represented by the given electron configuration?
Due to the phenomenon of half-orbital stability in the transition metals, electrons can easily move between 4s and 3d orbitals. The atom achieves greater stability from having only one atom in the 4s orbital, allowing a half-filled 3d orbital, as opposed to a full 4s orbital and four electrons in the 3d subshell.
For elements like chromium and copper, which could have valence shell configurations of 4s23d4 and 4s23d9, respectively, an electron from the 4s orbital jumps down to the 3d orbital to harness added stability from the half-filled orbital. The given electron configuration is that of chromium.
Note that you can also solve this question by counting the electrons to determine the atomic number. In this case, the electrons add up to 24, indicating the twenty-fourth element: chromium.
Example Question #1 : Electron Configuration
What is the correct electronic structure for 
Were this question asking for the electronic structure of magnesium (Mg) in its ground state, 




Note that the ground state of magnesium will have twelve electrons (the same as its atomic number), while the ion will have ten.
Certified Tutor
All GRE Subject Test: Chemistry Resources