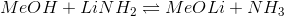All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #1 : Acid Base Reaction
For which of the following acid-base reactions will the equilibrium lie on the left side?
Possible Answers:

Correct answer:
Explanation:
The pKa value indicates how strong an acid is, and acid strength increases as pKa decreases. The side of a reaction with a lower pKa is going to dissociate more, pushing the equilibrium over to the other side. The equilibrium will thus lie on the side with the HIGHER pKa.
Since the pKa of acetic acid (4.76) is higher than the pKa of trifluoroacetic acid (0), the reaction will shift to the left to reach equilibrium.
Shivani
Certified Tutor
Certified Tutor
University of South Florida-Main Campus, Bachelor of Science, Cellular and Molecular Biology. University of South Florida-Mai...
All GRE Subject Test: Chemistry Resources
Popular Subjects
GMAT Tutors in San Diego, GMAT Tutors in Miami, French Tutors in Chicago, Computer Science Tutors in Seattle, Statistics Tutors in Philadelphia, Reading Tutors in Atlanta, Statistics Tutors in Seattle, Reading Tutors in San Diego, English Tutors in Atlanta, Reading Tutors in Washington DC
Popular Courses & Classes
SSAT Courses & Classes in Dallas Fort Worth, GMAT Courses & Classes in San Diego, MCAT Courses & Classes in San Francisco-Bay Area, LSAT Courses & Classes in Seattle, LSAT Courses & Classes in Los Angeles, MCAT Courses & Classes in Denver, SSAT Courses & Classes in Chicago, SAT Courses & Classes in Philadelphia, SAT Courses & Classes in Seattle, MCAT Courses & Classes in Miami
Popular Test Prep
LSAT Test Prep in Philadelphia, ISEE Test Prep in Dallas Fort Worth, SSAT Test Prep in Chicago, ISEE Test Prep in Boston, GRE Test Prep in Washington DC, SSAT Test Prep in Houston, ISEE Test Prep in Atlanta, LSAT Test Prep in Los Angeles, SAT Test Prep in Phoenix, MCAT Test Prep in Miami